Abstract
Background:
Spathe of phoenix dactylifera is hard-covering envelope of date palm which is mentioned as a nerve relief in ancient medicine books. In this experiment, three extract doses used in sleeping time, sedative efficacy, and electroencephalography (EEG) protocol to show different aspects of extract effects on sleep.
Materials and Methods:
In three sleeping time, anesthesia time and EEG experiment protocols test group containing three extract doses (62.5, 125, and 250 mg/kg) were compared with saline control group, and in sleeping time experiment control group contained intact, midazolam, and saline group to detail more in behavioral Angel method.
Results:
Three extract doses increased sleeping time when compared with saline control group (P < 0.05). In evaluated sedative efficacy, two 125 and 250 mg/kg doses increased anesthesia and showed sedative effect (P < 0.05). In EEG experiment, dose 125 mg/kg increased delta waves and decreased high-frequency waves of alpha and beta. In addition, there were significant decreases in alpha waves of 62.5 and 250 mg/kg doses.
Conclusion:
Although all three doses increased sleeping time, dose 125 mg/kg is more efficient for deep and relaxing sleep and suits more for related researches.
Keywords: Electroencephalography, insomnia, sedative and hypnotic, sleep
Introduction
Sleep serves a restorative function in the brain and is involved with memory retention. There are recurrent cycles of nonrapid eye movement (NREM) and rapid eye movement (REM) activity about every 90 min in normal sleep. Sleep stages can mentally and clinically show variance effects on human body,[1] specially slow-wave sleep (SWS) is important for consolidation of long-term memory.[2]
NREM sleep is divided into four stages: the first stage is the lightest, and fourth is the deepest stage of NREM sleep, the spectral composition of the electroencephalography (EEG) changes in different sleep stages.[3] Sleep deprivation plays an important role in health impairment[4,5] also correlates with mental distresses.[6]
Estimated prevalence of insomnia is about 10–40%.[7] About one-third of the adult population suffer from sleep problems and in some cases, medical treatment is necessary although most of the drugs which are used can intensify breathing disorders and sleep apnea that presents more in adults.[8] Benzodiazepines are a common group for sleep problems that have side effects of tolerance, withdrawal, complicate sleep behaviors, and cognitive disorders in some cases.
Spathe of phoenix dactylifera is a hard-covering envelope of male date palm. Researched article about this extract showed its analgesic effect,[9] Demirci et al. showed its repellent activity against the yellow fever mosquito,[10] Mokhtari and Sharifi showed its effect on histological changes on testis and luteinizing hormone, follicle-stimulating hormone, and testosterone concentrations,[11] Abdulla showed its antidiarrhoeal effect[12] and Hamedi et al. proposed different fractions of the spathe contain steroids, triterpene steroids, oils, and flavonoids.[13] In this research, doses were chosen according to analgesic research as some researchers showed same analgesic and hypnotic dose response in one same drug. In accordance with the researches and characteristics, the effect of hydroalcoholic spathe of phoenix dactylifera extract on sleeping time, sedative efficacy, and EEG brain waves were examined in this research.
Materials and Methods
Plants
Plants were gathered to be extracted by pharmacological maceration technique. The program of gathering spathe of phoenix dactylifera took place in Qeshm Island of Iran. All pieces have been dried and cleaned in room temperature and out of direct sun shine, and then 70% alcohol was added to the dried and grinded powder of spathe. For each 100 g of the powder, 400 ml of 70% alcohol was used and valves containing these solvent fastened to avoid alcohol evaporation. In order to reach complete, solvate containing alcohol shaked 2 hours on shaker system before percolating. Solvent condensed by rotary in 40°C and same temperature in bain-marie (water bath) for about 6 h followed by freeze drying of supernatant. The achieved extract solved by dimethyl sulfoxide in saline to obtain three doses of 62.5, 125, and 250 mg/kg.
Animal
For this experiment, Wistar male rats were used weighting about 270 ± 20 gathered from Isfahan University of Medical Sciences. Animals were kept in lucid cages in animal house at 24 ± 2°C with humidity 44–56%. There were light and dark cycle of 12 h beside reversed cycle that animal experienced light at 19 p.m. to 7 a.m. and rest times in dark. Lights were turned on and off automatically by the reverse set-up of the system. Rats diet were standard rodent pellet and water allowed ad libitum.
Protocol
Three protocols of sleeping time, sedative experiment, and EEG experiment were measured to assess different aspects of sleep and each group contained five rats. Sleeping time was measured by behavioral Angel method,[14] and rats were placed in sleep physiographic system. In sedative experiment, enhancing or reducing chloral hydrate anesthesia time was measured. In third protocol, animals were placed in EEG system for the evaluation of EEG brain wave activity to clarify extract effects on EEG brain waves. Animal behavioral methods were in accordance with medical university of Isfahan and International Animal Rules. Experimental groups had five members and were injected intraperitoneally. Temperature, lights, and other conditions were same for all groups.
Sleeping time
Rats usually have the sleep time of 10 a.m. to 16 p.m., 1 week before starting the protocol circadian rhythm of rat reversed. In this protocol, we used behavioral Angel method, rats placed in a special plastic box with a flexible metal plexus above it. This box is on two liquid-filled bags connected to a transducer that converts movements to electricity and a wire transfer it to physiograph. After 20 min of settling down and reducing stress, records started for 2 h. In this protocol, test group included three extract group and control include three salines, intact (without injection), and midazolam (positive control) groups 0.1 mg/kg.[15]
Sedative experiment
The sedative experiment included three extract doses for the test groups and a saline for the control group, test and control groups received extract and saline, respectively, after anesthetizing. Animals were anesthetized by 450 mg/kg chloral hydrate.
Extending anesthesia time by extract can show its sedative ability,[15] and reducing this time can shows adverse effect on sedative efficacy of extract groups. Rats placed in supine position in sleep system and checked period spending between anesthesia onset and recovery time. While recovering rat tries to change the upward position, so the different anesthesia time between groups leads to underestimating sedative effect of extract.
Brain wave
For this part of experiment, four groups were examined: saline control group and three test groups of extract doses 62.5, 125, and 250 mg/kg. After anesthetizing by urethane, animals were fixed on stereotaxic for surgery, 2 cranial holes were drilled over dura. Electrodes placed subcranially for EEG recording, in EEG system rats placed on a suitable pad and covered during the experiment to record better signals. Recording started as brain waves stabilized and three parts of 20 min recording has been recorded. No injection was accomplished for the first part (before part), but extract and saline were injected for test and control groups, respectively, in the second and third parts (after 1 and after 2 parts). Each of alpha, beta, delta, and theta waves was counted in percent of total power, for example, all waves gathered in after 1 wave were counted as 100% and every single wave was counted as a percentage of total power to show the results more clearly.
Statistical analysis
Results are shown as mean ± standard error of the mean and a significant difference was measured using ordinary one-way ANOVA and repeated-measure ANOVA with post t-test. Values were considered significant when P < 0.05. Statistical analyses were performed by statistical INSTAT software (GraphPad software Inc. GTA-32289-839)
Results
Sleeping time
Results of sleeping time are shown in Figure 1. All test groups receiving extract and midazolam group were significantly different from saline group. P value was more significant for midazolam positive control group (P < 0.001), and all extract groups were also meaningful (P < 0.05).
Figure 1.
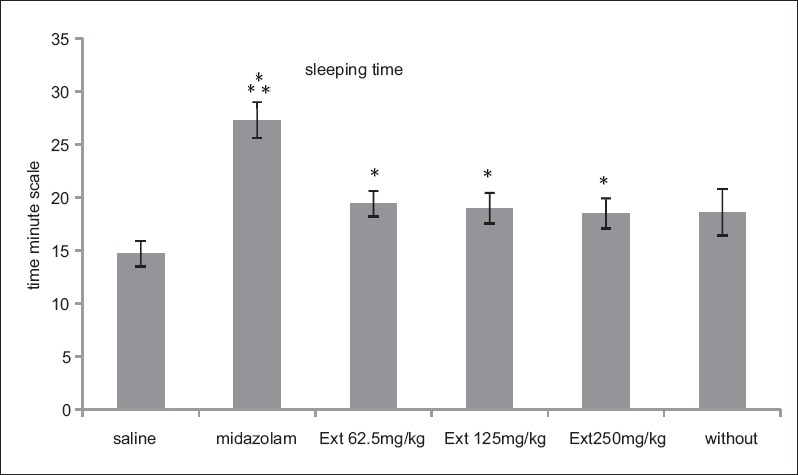
Rats sleeping time according to minute unite in three experimental groups of extract doses 62.5, 125, and 250 mg/kg and three control groups of saline, midazolam, and without injection. Midazolam and three extract groups increased sleeping time compared with saline control group. (***P < 0.001, *P < 0.05). Each bar represents mean ± standard error of the mean
Anesthesia time
To clarify the difference in four groups of sedated experiment, all groups were anesthetized; three groups receiving extract after anesthesia were compared with a saline control group to check its sedative efficacy; there are significant differences between groups. Four sedated groups are not similar as it is clear in Figure 2. Two groups of chloral hydrate and 62.5 mg/kg of tarooneh extract did not show any different effect, but there were significant differences among two other groups of 125 mg/kg and 250 mg/kg of extract with chloral hydrate with P < 0.001 and P < 0.01, respectively.
Figure 2.
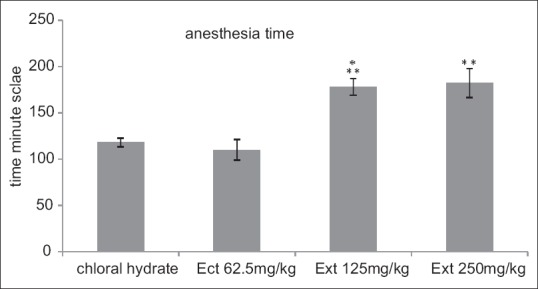
Rats anesthesia time according to minute unite in three experimental groups of extract doses 62.5, 125, and 250 mg/kg and control groups of chloral hydrate. 125 and 250 mg/kg groups increased anesthesia time compared with chloral hydrate control group. (***P < 0.001, **P < 0.01). Each bar represents mean ± standard error of the mean
Brain wave total power
In this experiment, percentage of waves counted to show extract effects on different waves. The percent of delta power is more than other waves in saline group and none of the waves of alpha, beta, theta, and delta changed significantly when compared to before wave [Figure 3].
Figure 3.
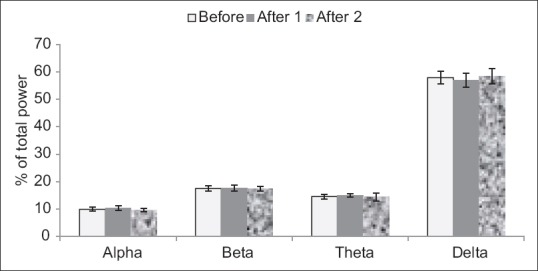
Saline injected for surveying electroencephalography brain waves. Each bar represents mean ± standard error of the mean
In dose 62.5 mg/kg and saline group delta power showed more amount in comparison to other waves; also there is a significant decrease in alpha power (P < 0.05) which is shown in Figure 4.
Figure 4.
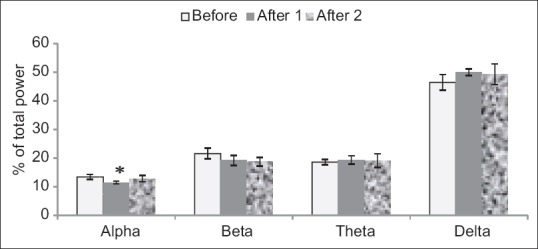
Injected dose 62.5 mg/kg of tarooneh extract for surveying electroencephalography brain waves. A significant decrease in alpha after 1 in comparison with before wave (*P < 0.05). Each bar represents mean ± standard error of the mean
In other dose experiment of 125 mg/kg, there was a very significant decrease in alpha after 2 in comparison with before wave (P < 0.001), it also decreased in comparing with after 2 (P < 0.05). Decrease in After 2 beta wave was significant in contrast to before wave of beta (P < 0.05) after 2 delta wave increased in comparison with before wave of delta (P < 0.05) [Figure 5].
Figure 5.
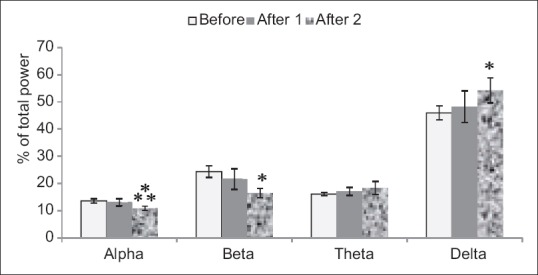
Injected dose 125 mg/kg of tarooneh extract for surveying electroencephalography brain waves. Significant decrease in alpha after 2 in compare with before wave, significant decrease in beta after 2 in compare with before wave and significant increase in delta after 2 in comparison with before wave (***P < 0.001, *P < 0.05). Each bar represents mean ± standard error of the mean
In experimented extract dose of 250 mg/kg, there was a significant decrease in alpha after 1 compared to before wave (P < 0.05) [Figure 6].
Figure 6.
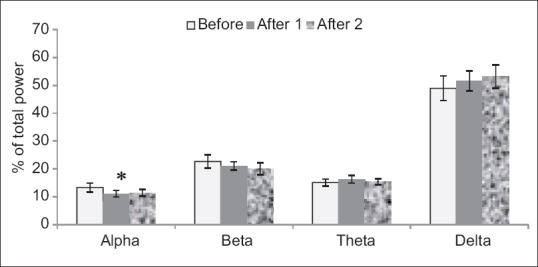
Injected dose 250 mg/kg of tarooneh extract for surveying electroencephalography brain waves. Significant decrease in alpha after 1 in comparison with before wave (*P < 0.05). Each bar represents mean ± standard error of the mean
Discussion
Sleep is controlled by neurologic functions[16] and can be monitored by the EEG system. This method is a good way of sleep monitoring[17] that shows different power waves. Theta and delta powers are two important parts of sleep stages that are more in stage 3 and stage 4; these two parts of sleep are called SWS that contain more delta power specially in stage 4.[18] SWS plays an important role in the restoration of cerebral functions and recovery in humans, also it has a very important role in maintenance and unification of sleep.[19] EEG delta wave also characterizes the NREM sleep in mammalian.[20] In deeper period of NREM sleep that delta power increases, it has been proposed that synaptic strength decreases to sustainable energy levels and causes synaptic plasticity and memory improvement.[21] Benzodiazepines function can increase NREM sleep also in another way it can decrease EEG delta power[22] while tumor necrosis factor, growth hormone releasing hormone, and interleukin-1 are cytokines that increase NREM along with an increase in EEG delta power.[23] There is evidence of cholinergic involvement in REM sleep in recent researches[24] cholinergic system can interfere with brain activity to control consciousness, and its activation can suppress slow wave activity and increase higher EEG frequency during sleep.[25] Some muscarinic antagonizing drugs can cause a reduced level of consciousness.[26]
This study along with focusing on sleeping time and sedative efficacy followed EEG brain waves to survey high-frequency waves in sleep that shows benefit effects on sleep quality.
Sleeping time
Results of behavioral Angel methods can show efficacy of tarooneh extract on sleeping time and increasing sleep tendency; also it shows the extract has less effect on sleeping tendency to compare to midazolam injected group.
Anesthesia time
It can show two 125 and 250 mg/kg doses are effective in anesthesia elongation, so these doses are considered to be sedative doses of extract according to increasing chloral hydrate anesthesia time.
Brain wave total power
There is a high delta power in all four EEG figures reflecting acquired anesthesia levels. In group 62.5 mg/kg decrease of after 1 alpha wave demonstrated its ability on reducing high-frequency waves and as a result increasing sleep tendency. Sharp effects of dose 125 mg/kg on decreasing high-frequency wave of alpha and beta are concomitant with increasing low-frequency wave of delta can show the sedative efficacy of this extract.
Increasing sleep tendency in dose 250 mg/kg is according to decreasing high-frequency wave of alpha. In spite of the fact that the sedative effects increased by increasing dose of 62.5 mg/kg to 125 mg/kg, the effects did not get more by increasing the dose to 250 mg/kg. The most effective dose of tarooneh extract is dose 125 mg/kg according to this research. The effects can be referred to flavonoid agents in the extract.[27]
Conclusion
Among three experimented extract doses, all were effective in sleeping time protocol and increased sleep time, but the purpose of sleep is more than sleep quantity. In the next two experiments, it was clear that dose 125 mg/kg of tarooneh extract can increase sleep quality along with sleep quantity so is more effective and relaxing.
Financial support and sponsorship
Authors are grateful to thank the Physiology and Pharmacognosy Department and the Deputy of Research, Isfahan University of Medical Sciences for financial support.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Authors are grateful to thank the Physiology and Pharmacognosy Department and the Deputy of Research, Isfahan University of Medical Sciences for financial support. This project is documented under registration no. 394114.
References
- 1.Lucey BP, Bateman RJ. Amyloid-ß diurnal pattern: Possible role of sleep in Alzheimer's disease pathogenesis. Neurobiol Aging. 2014;35(Suppl 2):S29–34. doi: 10.1016/j.neurobiolaging.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 2.Staner L. Sleep-wake mechanisms and drug discovery: Sleep EEG as a tool for the development of CNS-acting drugs. Dialogues Clin Neurosci. 2002;4:342–50. doi: 10.31887/DCNS.2002.4.4/lstaner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reinke L, van der Hoeven JH, van Putten MJ, Dieperink W, Tulleken JE. Intensive care unit depth of sleep: Proof of concept of a simple electroencephalography index in the non-sedated. Crit Care. 2014;18:R66. doi: 10.1186/cc13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickerson SS, Connors LM, Fayad A, Dean GE. Sleep-wake disturbances in cancer patients: Narrative review of literature focusing on improving quality of life outcomes. Nat Sci Sleep. 2014;6:85–100. doi: 10.2147/NSS.S34846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 6.Rosekind MR, Gregory KB, Mallis MM, Brandt SL, Seal B, Lerner D. The cost of poor sleep: Workplace productivity loss and associated costs. J Occup Environ Med. 2010;52:91–8. doi: 10.1097/JOM.0b013e3181c78c30. [DOI] [PubMed] [Google Scholar]
- 7.Uehli K, Mehta AJ, Miedinger D, Hug K, Schindler C, Holsboer-Trachsler E, et al. Sleep problems and work injuries: A systematic review and meta-analysis. Sleep Med Rev. 2014;18:61–73. doi: 10.1016/j.smrv.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Mignot E, Taheri S, Nishino S. Sleeping with the hypothalamus: Emerging therapeutic targets for sleep disorders. Nat Neurosci. 2002;5:1071–5. doi: 10.1038/nn944. [DOI] [PubMed] [Google Scholar]
- 9.Dashti M, Vahidi A, Panjalizadeh A. Effect of phoenix dactylifera spathe hydroalcoholic extract on chronic pain in mice. J Med Plants. 2012;11:136–44. [Google Scholar]
- 10.Demirci B, Tsikolia M, Bernier UR, Agramonte NM, Alqasoumi SI, Al-Yahya MA, et al. Phoenix dactylifera L. spathe essential oil: Chemical composition and repellent activity against the yellow fever mosquito. Acta Tropica. 2013;128:557–60. doi: 10.1016/j.actatropica.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Mokhtari M, Sharifi E. Effect of alcoholic extract of phoenix dactylifera spathe on histological change in testis and concentrations of LH, FSH and testosterone in male rat. Iran J Basic Med Sci. 2007;4:265–71. [Google Scholar]
- 12.Al-Taher AY. Possible anti-diarrhoeal effect of the date palm (Phoenix dactylifera L) spathe aqueous extract in rats. Sci J King Faisal Univ. 2008;9:131–8. [Google Scholar]
- 13.Hamedi A, Mohagheghzadeh A, Rivaz S. Preliminary pharmacognostic evaluation and volatile constituent analysis of spathe of Phoenix dactylifera L.(tarooneh) Pharmacogn J. 2013;5:83–6. [Google Scholar]
- 14.Jadidi M, Vafaee AA, Miladigorji H, Moghimi HR, Amiri M. Surveying the effect of Ferula persica L on symptom of morphin withdrawal and sleep in mice. Gorgan Health Mag. 2009;34:225–30. [Google Scholar]
- 15.Koch S, Fitzgerald M, Hathway G. Midazolam potentiates nociceptive behavior, sensitizes cutaneous reflexes, and is devoid of sedative action in neonatal rats. Am Soc Anesthesiol. 2008;108:122–9. doi: 10.1097/01.anes.0000296079.45446.15. [DOI] [PubMed] [Google Scholar]
- 16.Cipriani G, Lucetti C, Danti S, Nuti A. Sleep disturbances and dementia. Psychogeriatrics. 2015;15:65–74. doi: 10.1111/psyg.12069. [DOI] [PubMed] [Google Scholar]
- 17.Luan B, Jia W, Thirumala PD, Balzer J, Gao D, Sun M. A feasibility study on a single-unit wireless EEG sensor. Int Conf Signal Process Proc. 2014;2014:2282–5. doi: 10.1109/ICOSP.2014.7015401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth TH. Slow wave sleep: Does it matter. J Clin Sleep Med. 2009;5:S4–5. [PMC free article] [PubMed] [Google Scholar]
- 19.Dijk DJ, Groeger J, Deacon S, Stanley N. Association between individual differences in slow wave sleep, slow wave activity and sleep continuity in young, middle-aged and older men and women. Eur Neuropsychopharmacol. 2006;16:S538. [Google Scholar]
- 20.Davis CJ, Clinton JM, Jewett KA, Zielinski MR, Krueger JM. Delta wave power: An independent sleep phenotype or epiphenomenon? J Clin Sleep Med. 2011;7 5 Suppl:S16–8. doi: 10.5664/JCSM.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Bastien CH, LeBlanc M, Carrier J, Morin CM. Sleep EEG power spectra, insomnia, and chronic use of benzodiazepines. Sleep. 2003;26:313–7. doi: 10.1093/sleep/26.3.313. [DOI] [PubMed] [Google Scholar]
- 23.Obal F, Jr, Krueger JM. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci. 2003;8:520–50. doi: 10.2741/1033. [DOI] [PubMed] [Google Scholar]
- 24.Hobson JA, Stickgold R, Pace-Schott EF. The neuropsychology of REM sleep dreaming. Neuroreport. 1998;9:R1–14. doi: 10.1097/00001756-199802160-00033. [DOI] [PubMed] [Google Scholar]
- 25.Seifritz E. Contribution of sleep physiology to depressive pathophysiology. Neuropsychopharmacology. 2001;25 5 Suppl:S85–8. doi: 10.1016/S0893-133X(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 26.Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: A neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22:273–80. doi: 10.1016/s0166-2236(98)01361-7. [DOI] [PubMed] [Google Scholar]
- 27.Fang XS, Hao JF, Zhou HY, Zhu LX, Wang JH, Song FQ. Pharmacological studies on the sedative-hypnotic effect of Semen Ziziphi spinosae (Suanzaoren) and Radix et Rhizoma Salviae miltiorrhizae (Danshen) extracts and the synergistic effect of their combinations. Phytomedicine. 2010;17:75–80. doi: 10.1016/j.phymed.2009.07.004. [DOI] [PubMed] [Google Scholar]


