Significance
Maternal immune activation (MIA) plays a role in neurodevelopmental disorders such as schizophrenia and autism spectrum disorder (ASD); however, the precise mechanisms of its etiology remain largely unknown. Soluble epoxide hydrolase (sEH) plays a role in the inflammation associated with MIA. Expression of sEH protein in the prefrontal cortex of juvenile offspring after MIA was higher than controls. Expression of sEH mRNA in neurospheres from induced pluripotent stem cells of schizophrenia patients with the 22q11.2 deletion and in the postmortem brains from ASD patients was higher than controls. Treatment with sEH inhibitor could prevent the onset of behavioral abnormalities in offspring after MIA. Our findings indicate that sEH inhibitors are promising prophylactic or therapeutic drugs for neurodevelopmental disorders in offspring after MIA.
Keywords: epoxy fatty acid, ER stress, iPSCs, maternal infection, prevention
Abstract
Maternal infection during pregnancy increases risk of neurodevelopmental disorders such as schizophrenia and autism spectrum disorder (ASD) in offspring. In rodents, maternal immune activation (MIA) yields offspring with schizophrenia- and ASD-like behavioral abnormalities. Soluble epoxide hydrolase (sEH) plays a key role in inflammation associated with neurodevelopmental disorders. Here we found higher levels of sEH in the prefrontal cortex (PFC) of juvenile offspring after MIA. Oxylipin analysis showed decreased levels of epoxy fatty acids in the PFC of juvenile offspring after MIA, supporting increased activity of sEH in the PFC of juvenile offspring. Furthermore, expression of sEH (or EPHX2) mRNA in induced pluripotent stem cell-derived neurospheres from schizophrenia patients with the 22q11.2 deletion was higher than that of healthy controls. Moreover, the expression of EPHX2 mRNA in postmortem brain samples (Brodmann area 9 and 40) from ASD patients was higher than that of controls. Treatment with 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl)urea (TPPU), a potent sEH inhibitor, in juvenile offspring from prenatal day (P) 28 to P56 could prevent cognitive deficits and loss of parvalbumin (PV) immunoreactivity in the medial PFC of adult offspring after MIA. In addition, dosing of TPPU to pregnant mothers from E5 to P21 could prevent cognitive deficits, and social interaction deficits and PV immunoreactivity in the medial prefrontal cortex of juvenile offspring after MIA. These findings suggest that increased activity of sEH in the PFC plays a key role in the etiology of neurodevelopmental disorders in offspring after MIA. Therefore, sEH represents a promising prophylactic or therapeutic target for neurodevelopmental disorders in offspring after MIA.
Epidemiological studies implicate prenatal environmental factors, including maternal immune activation (MIA), in playing a key role in the etiology of neurodevelopmental disorders such as schizophrenia and autism spectrum disorder (ASD) (1–7). A number of studies suggest associations between maternal infections or inflammatory biomarkers and schizophrenia and ASD (2–4, 7). For example, there are key epidemiological results supporting associations between maternal infectious pathogens (i.e., influenza virus, herpes simplex virus, Toxoplasma gondii, rubella, and bacterial pathogens) and inflammatory biomarkers (i.e., cytokines and C-reactive protein) and schizophrenia (2, 7, 8). The Finnish Prenatal Studies birth cohort showed that elevated maternal levels of C-reactive protein in early to midgestation was related to an increased risk of ASD in offspring (9), although maternal midpregnancy levels of C-reactive protein were related to a decreased risk of ASD (10). A meta-analysis suggests that maternal infection during pregnancy increases the risk of ASD in offspring (4). Collectively, MIA during pregnancy can increase the risk of neurodevelopmental disorders in offspring. The onset of schizophrenia and ASD is in young adulthood and before 3 y of age, respectively. However, the precise mechanisms underlying MIA-induced increase of the risk for neurodevelopmental disorders remain largely unknown.
Although animal models are limited in their generalizability to neurodevelopmental disorders, accumulating studies demonstrate the neurobiological pathways between MIA and neurodevelopmental disorders (7). A number of studies make use of immune-activating agents that primarily stimulate the innate immune system, such as the synthetic double-stranded RNA analog polyriboinosinic-polyribocytidilic acid [poly(I:C)], a Toll-like receptor 3 agonist (11–15). Offspring of prenatal rodents exposed to poly(I:C) mimic schizophrenia (or ASD)-like behavioral abnormalities in adulthood (or childhood), although the MIA model using poly(I:C) does not reproduce the full spectrum of immune responses normally induced by infectious pathogens (7).
Many epoxy fatty acids (EpFAs) are produced from the corresponding olefins by cytochrome P450 enzymes. Epoxyeicosatrienoic acids (EETs) and epoxydocosapentaenoic acids (EDPs) are produced from arachidonic acid and docosahexaenoic acid (DHA), respectively. EETs, EDPs, and some other EpFAs have potent antiinflammatory properties. However, these mediators are broken down into their corresponding diols by soluble epoxide hydrolase (sEH), and inhibition of sEH enhances the beneficial effects of EpFAs such as EETs (16–18). Potent antiinflammatory effects of EETs and key role of sEH have been reported in multiple animal models, including pain, obesity, depression, and Parkinson’s disease (19–27). However, there are no reports showing the role of sEH in the pathogenesis of neurodevelopmental disorders in offspring after MIA.
The purpose of this study was to examine the role of sEH in the pathogenesis of neurodevelopmental disorders in offspring after MIA. First, we examined whether expression of sEH is altered in the brain regions of juvenile offspring after neonatal poly(I:C) exposure. Next, we performed oxylipin analysis of brain regions from juvenile offspring after MIA. Second, using induced pluripotent stem cells (iPSCs), we examined whether expression of sEH (or EPHX2) mRNA is altered in the neurospheres from iPSCs from patients with schizophrenia with the 22q11.2 deletion since patients with the microdeletion exhibit a spectrum of cognitive deficits, and ∼30% of them develop typical schizophrenia in adolescence or early adulthood (28, 29). Furthermore, we measured the expression of EPHX2 mRNA in postmortem brain samples from ASD patients, as well as age-matched control subjects. Third, we examined whether 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl)urea (TPPU), a potent sEH inhibitor (22, 30, 31), during juvenile and adolescent stages could prevent behavioral abnormalities and reduction of parvalbumin (PV) and glutamic acid decarboxylase (GAD67) immunoreactivity (IR) in the medial prefrontal cortex (mPFC) in adult offspring after MIA. Furthermore, the effects of TPPU on endoplasmic reticulum (ER) stress in the brain from adult offspring after MIA were also examined. Finally, we examined whether treatment of TPPU in pregnant mice from pregnancy to weaning could prevent behavioral abnormalities in juvenile offspring after MIA.
Results
Levels of sEH and Eicosanoid Metabolites in the Brain from Juvenile Offspring After MIA.
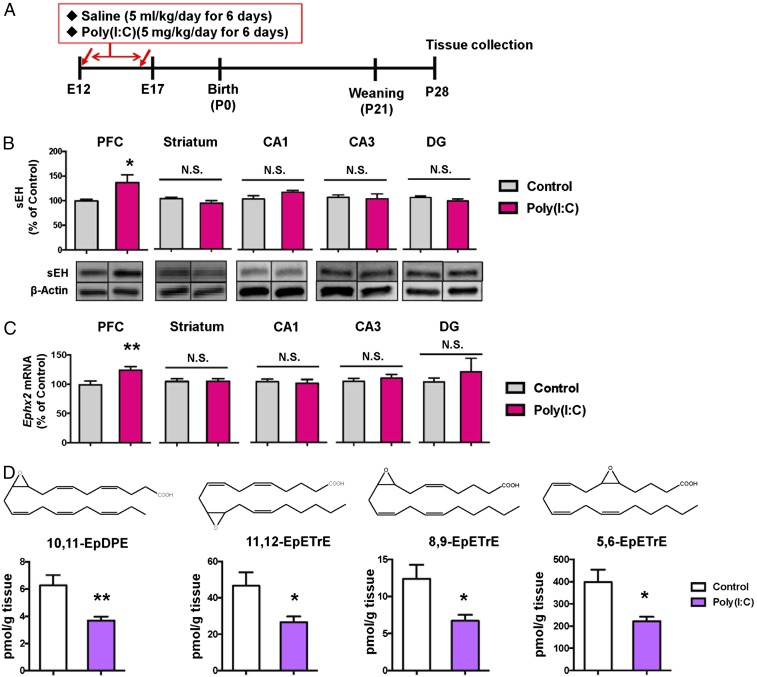
First, we examined whether expressions of sEH are altered in the brain regions from juvenile offspring of mice after neonatal treatment with poly(I:C) (5 mg/kg/d from E12 to E17) (Fig. 1A). Levels of sEH in the PFC from juvenile offspring from poly(I:C)-treated mice are significantly higher than those of saline-treated mice (Fig. 1B). In contrast, there were no changes of sEH in other brain regions such as striatum and hippocampus (CA1, CA3, dentate gyrus) (Fig. 1B). Furthermore, expressions of sEH (or Ephx2) mRNA in the PFC, but not in other regions, from juvenile offspring from poly(I:C)-treated mice are significantly higher than those of saline-treated mice (Fig. 1C).
Fig. 1.
Increased expression of sEH and decreased levels of epoxy fatty acids in the brain from juvenile offspring after MIA. (A) Schedule of treatment and brain collection. (B) Protein expression of sEH in the mouse brain regions from juvenile offspring after prenatal poly(I:C) exposure. Data are shown as mean ± SEM (n = 7 or 8). (C) Gene expression of Ephx2 mRNA in the mouse brain regions from juvenile offspring after prenatal poly(I:C) exposure. Data are shown as mean ± SEM (n = 6). (D) Tissue levels of four EpFAs such as 10,11-EpDPE; 11,12-EpETrE; 8,9-EpETrE; and 5,6-EpETrE in the PFC from juvenile offspring after MIA. The values represent the mean ± SEM. (n = 10 or 11). *P < 0.05, **P < 0.01 compared with control group (two-tailed Student t test). N.S, not significant.
Using oxylipin analysis, we measured tissue levels of eicosanoid metabolites in the PFC, hippocampus, and cerebellum from juvenile offspring after neonatal poly(I:C) (or saline) exposure (SI Appendix, Fig. S1 and Tables S1–S3). Tissue levels of 10,11-EpDPE (10,11-epoxy-4Z,7Z,13Z,16Z,19Z-docosapentaenoic acid), 11,12-EpETrE [(5Z,8Z,14Z)-11,12-epoxyicosa-5,8,14-trienoate], 8,9-EpETrE (8,9-epoxy-5Z,11Z,14Z-eicosatrienoic acid), 5,6-EpETrE [N-((+/−)-5,6-epoxy-8Z,11Z,14Z-eicosatrienoyl)-ethanolamine], and 6-keto-PGF1α in the PFC from juvenile offspring after MIA were significantly lower than those of control mice (Fig. 1D and SI Appendix, Table S1). In contrast, tissue levels of 19,20-DiHDPE [(4Z,7Z,10Z,13Z,16Z)-19,20-dihydroxydocosa-4,7,10,13,16-pentaenoic acid] in the PFC from juvenile offspring after MIA were significantly higher than those of control mice. Lower levels of these epoxy eicosanoids (10,11-EpDPE; 11,12-EpETrE; 8,9-EpETrE; 5,6-EpETrE) in the PFC from juvenile offspring after MIA support the increased expression of sEH in this region. Furthermore, tissue levels of thromboxane B2, 19,20-DiHDPE, EKODE [12,13-epoxy 9-keto-10(trans)-octadecenoic acid], and 9-oxo-ODE (9-oxo-10E,12Z-octadecadienoic acid) in the hippocampus from juvenile offspring after MIA were significantly higher than those of control mice (SI Appendix, Table S2). In contrast, there were no changes of eicosanoid metabolites in the cerebellum between two groups (SI Appendix, Table S3).
Increased Expression of sEH in the Neurospheres from iPSCs from Patients with Schizophrenia and Postmortem Brain Samples from ASD Patients.
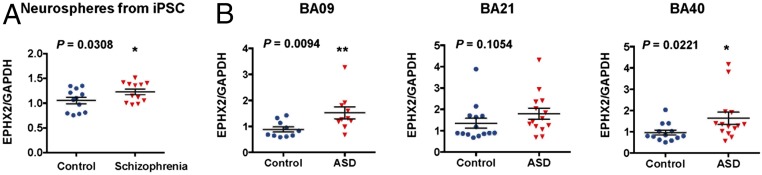
iPSC technologies have provided an unprecedented opportunity to establish pluripotent stem cells from patients with schizophrenia and differentiate them into a neuronal lineage, enabling an in vitro recapitulation of the pathogenesis of the disease (32). Previously, we reported that expression of sEH in the parietal cortex [Brodmann area (BA) 7] from schizophrenia patients was significantly higher than that of controls (22). Therefore, we measured whether sEH gene expression alters in the neurospheres from iPSCs from schizophrenia patients with the 22q11.2 deletion (SI Appendix, Table S4) and healthy controls (33). Expression of EPHX2 mRNA in the neurospheres from iPSCs with schizophrenia patients was significantly higher than that of healthy control subjects (Fig. 2A).
Fig. 2.
Increased expression of EPHX2 mRNA in the neurospheres from iPSC of schizophrenia patients and postmortem brain samples from ASD patients. (A) Gene expression of EPHX2 mRNA in the neurospheres from iPSC from schizophrenia patients with the 22q11.2 deletion was significantly higher than that of healthy controls. Data are shown as mean ± SD (n = 12). *P < 0.05 compared with control group (one-tailed Student t test). (B) Gene expression of EPHX2 mRNA in the BA09 and BA40, but not BA21, from ASD patients was significantly higher than that of controls. Data are shown as mean ± SD; *P < 0.05 and **P < 0.01 compared with control group (one-tailed Student t test).
Next, we measured expression of EPHX2 mRNA in the postmortem brain samples (BA09, BA21, BA40) from ASD patients and age- and gender-matched controls (SI Appendix, Table S5) (34). Expression of EPHX2 mRNA in the BA09 and BA40 from ASD patients was significantly higher than that of controls (Fig. 2 B and D). Expression of EPHX2 mRNA in the BA21 from ASD patients was slightly higher than that of controls, but statistical analysis did not reach statistical significance (Fig. 2C). Collectively, it is likely that increased expression of sEH may play a role in the pathogenesis of schizophrenia and ASD.
Effects of TPPU Treatment During Juvenile and Adolescent Stages on Cognitive Deficits and Reduction of PV- and GAD67-IR in the mPFC of Adult Offspring After MIA.
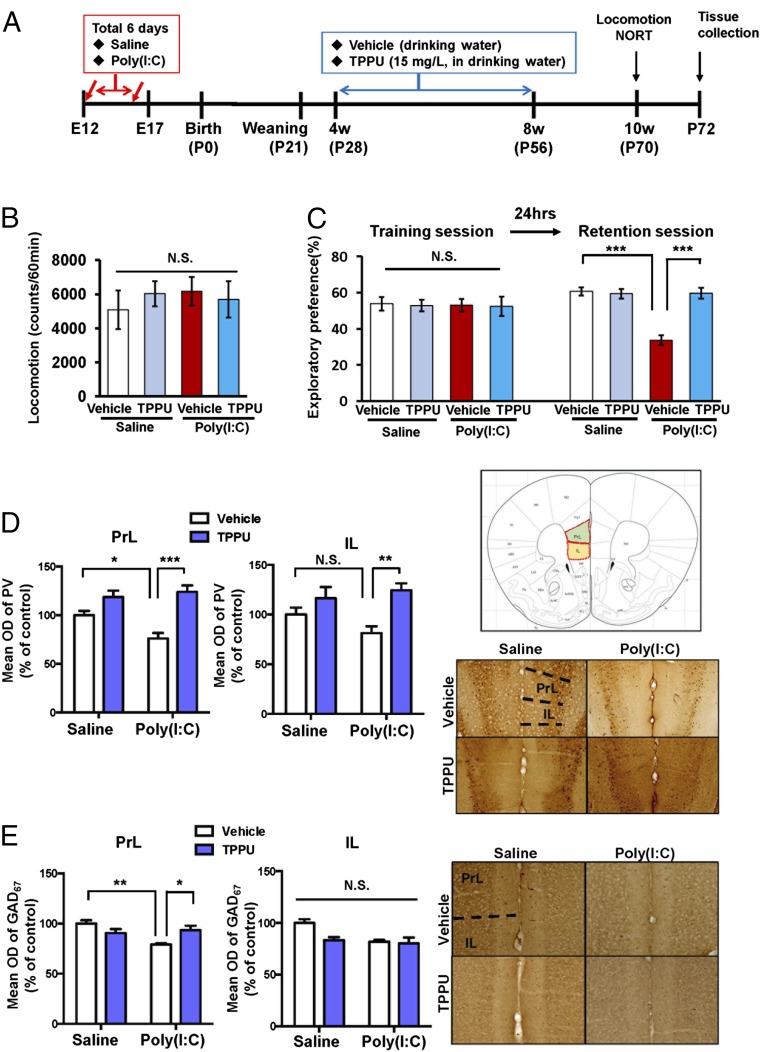
Cognitive impairment is the core symptom in patients with schizophrenia (35). Previously, we reported that juvenile offspring of prenatal poly(I:C)-treated mice showed cognitive deficits and the reduction of PV-IR in the mPFC (36–39). Here we examined whether TPPU (15 mg/L) in drinking water during juvenile and adolescent stages [from prenatal day (P) 28 to P56] could prevent cognitive deficits and reduction of PV-IR in the mPFC of adult offspring after MIA. In the open field test, locomotion was unchanged among the four groups (Fig. 3B). There was no difference among the four groups in training sessions of the novel object recognition test (NORT). However, in the retention session of NORT, the exploratory preference of the poly(I:C) + TPPU group was significantly higher than that of the poly(I:C) + water group (Fig. 3C). There were no changes in the body weight among the four groups (SI Appendix, Fig. S2). These findings suggest that TPPU in drinking water from P28 to P56 could improve cognitive deficits in adult offspring after MIA.
Fig. 3.
Effects of TPPU on behavioral abnormalities and the reduction of PV-IR and GAD67-IR in the mPFC of adult offspring after MIA. (A) Schedule of treatment and brain collection. Saline or poly(I:C) (5 mg/kg/d for 6 d) was administered i.p. to pregnant mice. Vehicle or TPPU (15 mg/L) in drinking water was administered to juvenile offspring from P28 to P56. Subsequently, all mice received normal water. Locomotion and NORT were performed when the mice were 10 wk old. Brain for immunohistochemistry was collected at P72. (B) Locomotion test. (C) NORT. (D) PV immunohistochemistry. (E) GAD67 immunohistochemistry. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with poly(I:C) + vehicle group. IL: infralimbic of medial prefrontal cortex. N.S, not significant. PrL, prelimbic of medial prefrontal cortex. Detailed statistical analysis data are in SI Appendix, Table S6.
Furthermore, we performed PV and GAD67 immunohistochemistry at adulthood (10 wk) (Fig. 3D). PV-IR in the prelimbic (PrL), not IL (infralimbic), of mPFC in the offspring of poly(I:C)-treated mice was significantly lower than that of the saline-treated group (Fig. 3D). PV-IR in the PrL (not IL) of the mPFC of the poly(I:C) + TPPU group was significantly higher than that in the poly(I:C) + control group (Fig. 3D).
GAD67, a key enzyme of γ-aminobutyric acid synthesis, is reported to be lower in the PFC from schizophrenic patients (40, 41). GAD67-IR in the PrL and IL of mPFC in the offspring of poly(I:C)-treated mice was significantly lower than that of the saline-treated group (Fig. 3E). GAD67-IR in the PrL (not IL) of the mPFC of the poly(I:C) + TPPU group was significantly higher than that in the poly(I:C) + control group (Fig. 3E). These findings suggest that TPPU in drinking water from P28 to P56 could prevent the reduction of PV- and GAD67-IR in the PrL of the mPFC in adult offspring after MIA.
Role of sEH in ER Stress in the Mouse Brain from Juvenile Offspring After MIA.
It is reported that the sEH inhibitor attenuates activation of the ER (26, 42–44). In this study, we examined the effects of TPPU on ER stress in the brain regions from juvenile offspring after MIA. We found increased levels of three key sensors in the ER stress-signaling pathway, including PKR-like ER-resident kinase (PERK), inositol-requiring enzyme 1α (IRE1α), and activating transcription factor 6 (ATF6) (SI Appendix, Fig. S3 A and B). Levels of the associated downstream targets were elevated, suggesting full-scale activation of the ER stress pathway (26, 43). Accordingly, phosphorylation of eukaryotic initiation factor 2 subunit α (eIF2α), mediated by phospho-PERK, was also increased. Phosphorylation of IRE1α led to a significant rise in total protein levels of spliced X-box–binding protein 1 (Xbp1), as well as levels of the ER chaperone-binding Ig protein (Bip). Increased phosphorylation of p38 and c-jun NH2-terminal kinase (JNK) 1/2 was also observed. Pharmacological inhibition by TPPU significantly attenuated ER stress in the brain regions from offspring after MIA (SI Appendix, Fig. S3 A and B).
Effect of TPPU in Drinking Water on Cognitive and Social Interaction Deficits and in Juvenile Offspring of Prenatal Mice Exposed to Poly(I:C).
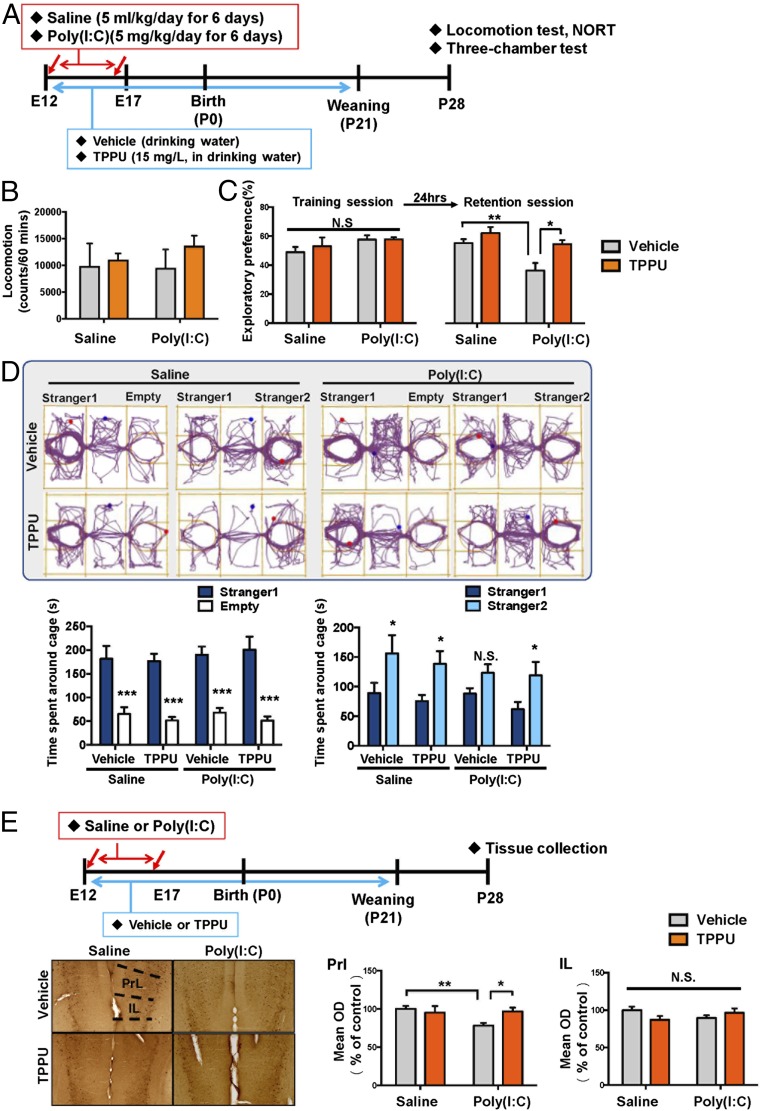
The pregnant mice were administrated with vehicle or TPPU (15 mg/L in drinking water) from E12 to 3-wk-old mice (P21). Subsequently, normal drinking water was given to all male offspring for an additional 2 wk (from 3 to 5 wk old). Behavioral tests were performed at 4–5 wk of age (Fig. 4A). There were no differences of locomotion among the four groups (Fig. 4B). Two-way ANOVA analysis of NORT data in the training session revealed no significant interaction among four groups (Fig. 4C). In the retention session of NORT, two-way ANOVA analysis revealed a significant effect among four groups (Fig. 4C). Exploratory preference of poly(I:C) + water group was significantly lower than that of saline + water group or poly(I:C) + TPPU group (Fig. 4C).
Fig. 4.
Effects of TPPU on behavioral abnormalities and the reduction of PV-IR in the mPFC of juvenile offspring after MIA. (A) Schedule of treatment and brain collection. Saline or poly(I:C) (5 mg/kg/d for 6 d) was administered i.p. into pregnant mice. Vehicle or TPPU (15 mg/L) in drinking water was administered to pregnant mice from E12 to P21. Subsequently, all mice received normal water. Locomotion and NORT were performed after P28. Brain tissue for immunohistochemistry was collected after behavioral tests. (B) Locomotion test. (C) NORT. (D) Three-chamber social interaction test. (E) PV immunohistochemistry. Decreased PV-IR in the PrL of mPFC of juvenile offspring after prenatal poly(I:C) exposure was significantly attenuated by TPPU in drinking water. Data are shown as mean ± SEM (n = 6–8). *P < 0.05, **P < 0.01, and ***P < 0.001, compared with poly(I:C) + vehicle group. IL, infralimbic of medial prefrontal cortex. N.S, not significant. PrL, prelimbic of medial prefrontal cortex. Detailed statistical analysis data are in SI Appendix, Table S6.
In the three-chamber social interaction test, TPPU in drinking water significantly improved social interaction deficits in juvenile offspring after MIA (Fig. 4D). Furthermore, TPPU in drinking water significantly attenuated reduction of PV-IR in the PrL in the mPFC of juvenile offspring after MIA (Fig. 4E).
Discussion
The present results demonstrate a key role of sEH in the pathogenesis of neurodevelopmental disorders in offspring after MIA. The major findings of the present study are as follows: First, expression of sEH protein in the PFC from juvenile offspring after MIA was higher than that of control group. Oxylipin analysis showed lower levels of EpFAs in the PFC from juvenile offspring after MIA, supporting higher levels of sEH in this region. Second, we found higher expression of EPHX2 mRNA in the neurospheres from iPSC of schizophrenia patients compared with healthy controls. In addition, we found higher expression of EPHX2 mRNA in the postmortem brain samples from ASD patients compared with control group. Third, TPPU in drinking water during the juvenile and adolescent stages of offspring after MIA prevented cognitive deficits and reduction of PV-IR and GAD67-IR in the PrL of the mPFC at adulthood after MIA. Furthermore, TPPU in drinking water during the juvenile and adolescent stages of offspring after MIA significantly attenuated ER stress in the PFC from adult offspring after MIA. Finally, TPPU in drinking water in poly(I:C)-treated pregnant mice from pregnancy (E12) to weaning (P21) could prevent the onset of cognitive deficits and social interaction deficits and reduction of PV-IR in the mPFC in juvenile offspring after MIA. Collectively, these findings suggest that sEH plays a key role in the pathogenesis of neurodevelopmental disorders such as schizophrenia and ASD and that sEH inhibitors may prove to be promising prophylactic or therapeutic drugs for these disorders.
In this study, we found increased expression of sEH protein in the PFC of juvenile offspring after prenatal poly(I:C) exposure, although expression of sEH in other regions (striatum and hippocampus) remained the same. Thus, it seems that increases in the sEH in the PFC might play a role in the behavioral and biochemical abnormalities seen in juvenile offspring after MIA. Previously, we reported increased levels of sEH in the parietal cortex from schizophrenia patients compared with controls (22). In this study, we found higher levels of EPHX2 mRNA in the neurospheres from iPSC from schizophrenia patients and in the postmortem brain samples from ASD patients. These findings suggest that increased metabolism of EpFAs in the corresponding diols by increased sEH may play a role in the pathogenesis of schizophrenia and ASD, although further detailed studies on how prenatal poly(I:C) exposure induces abnormalities in the eicosanoid metabolism by sEH and behavioral abnormalities in juveniles and adults are needed.
Tissue levels of EpFAs were significantly lower in the PFC from juvenile offspring after MIA than in those of control mice, supporting an increased activity of sEH in the PFC. The EpFAs such as 11,12-EpETrE, 8,9-EpETrE, and 5,6-EpETrE are metabolized to its corresponding diol, dihydroxyeicosatrienoic acid, by sEH (Fig. 1D). It is known that EETs such as EpETrE are important components of many intracellular signaling pathways in both cardiac and extracardiac tissues (45) and that EETs and some other EpFAs possess potent antiinflammatory properties (23, 46). Although the precise mechanisms underlying the relationship between EpETrEs and sEH in the PFC from juvenile offspring after MIA are currently unclear, it seems that increased metabolism of 10,12-EpETrE, 8,9-EpETrE, and 5,6-EpETrE by increased levels of sEH in the PFC may be involved in behavioral abnormalities of offspring after MIA. By contrast, we found increased levels of EKODE in the hippocampus of juvenile offspring after MIA although levels of sEH were not altered. Although the reasons underlying this discrepancy are currently unknown, it seems that multiple pathways may contribute to formation and degradation of EKODE in the hippocampus. Further detailed study on the metabolism of EKODE in neurodevelopmental disorders is needed (SI Appendix, Supplementary Information Text).
In conclusion, these findings suggest that increased sEH and subsequent decreased EpFAs might play a key role in the etiology of neurodevelopmental disorders in offspring after MIA. Therefore, sEH inhibitors appear to be prophylactic or therapeutic drugs for MIA-related neurodevelopmental disorders such as schizophrenia and ASD.
Materials and Methods
Details of the experimental protocols, including animals, materials, MIA model, Western blot analysis, RT-PCR, oxylipin analysis, human iPSCs, immunohistochemistry, and statistical analysis are given in SI Appendix.
Supplementary Material
Acknowledgments
This study was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grants 18K15439 (to Q.R.) and 17H04243 (to K.H.); Japan Agency for Medical Research and Development Grants JP18dm0107119 (to K.H.) and JP18dm0107083 (to T.Y.); the Ministry of Education, Culture, Sports, Science and Technology, Japan Grant JP18H05435 (to T.Y.); National Institute of Environmental Health Sciences (NIEHS) Grant R01 ES002710 (to B.D.H.); and NIEHS Superfund Program Grant P42 ES004699 (to B.D.H.). M.M. was supported by the Nurture of Creative Research Leaders in Immune System Regulation and Innovative Therapeutics Program of Chiba University and by a Research Fellowship for Young Scientists of JSPS.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1819234116/-/DCSupplemental.
References
- 1.Brown AS, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 2.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: A review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estes ML, McAllister AK. Maternal immune activation: Implications for neuropsychiatric disorders. Science. 2016;353:772–777. doi: 10.1126/science.aag3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang HY, et al. Maternal infection during pregnancy and risk of autism spectrum disorders: A systematic review and meta-analysis. Brain Behav Immun. 2016;58:165–172. doi: 10.1016/j.bbi.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Careaga M, Murai T, Bauman MD. Maternal immune activation and autism spectrum disorder: From rodents to nonhuman and human primates. Biol Psychiatry. 2017;81:391–401. doi: 10.1016/j.biopsych.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zerbo O, et al. Association between influenza infection and vaccination during pregnancy and risk of autism spectrum disorder. JAMA Pediatr. 2017;171:e163609. doi: 10.1001/jamapediatrics.2016.3609. [DOI] [PubMed] [Google Scholar]
- 7.Brown AS, Meyer U. Maternal immune activation and neuropsychiatric illness: A translational research perspective. Am J Psychiatry. 2018;175:1073–1083. doi: 10.1176/appi.ajp.2018.17121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canetta S, et al. Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Am J Psychiatry. 2014;171:960–968. doi: 10.1176/appi.ajp.2014.13121579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown AS, et al. Elevated maternal C-reactive protein and autism in a national birth cohort. Mol Psychiatry. 2014;19:259–264. doi: 10.1038/mp.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zerbo O, et al. Maternal mid-pregnancy C-reactive protein and risk of autism spectrum disorders: The early markers for autism study. Transl Psychiatry. 2016;6:e783. doi: 10.1038/tp.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: A novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- 12.Ozawa K, et al. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: A neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 13.Patterson PH. Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behav Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Meyer U. Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry. 2014;75:307–315. doi: 10.1016/j.biopsych.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Gumusoglu SB, Stevens HE. Maternal inflammation and neurodevelopmental programming: A review of preclinical outcomes and implications for translational psychiatry. Biol Psychiatry. 2019;85:107–121. doi: 10.1016/j.biopsych.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Morisseau C, Hammock BD. Epoxide hydrolases: Mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol. 2005;45:311–333. doi: 10.1146/annurev.pharmtox.45.120403.095920. [DOI] [PubMed] [Google Scholar]
- 17.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner K, Vito S, Inceoglu B, Hammock BD. The role of long chain fatty acids and their epoxide metabolites in nociceptive signaling. Prostaglandins Other Lipid Mediat. 2014;113–115:2–12. doi: 10.1016/j.prostaglandins.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.López-Vicario C, et al. Inhibition of soluble epoxide hydrolase modulates inflammation and autophagy in obese adipose tissue and liver: Role for omega-3 epoxides. Proc Natl Acad Sci USA. 2015;112:536–541. doi: 10.1073/pnas.1422590112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren Q, et al. Gene deficiency and pharmacological inhibition of soluble epoxide hydrolase confers resilience to repeated social defeat stress. Proc Natl Acad Sci USA. 2016;113:E1944–E1952. doi: 10.1073/pnas.1601532113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashimoto K. Soluble epoxide hydrolase: A new therapeutic target for depression. Expert Opin Ther Targets. 2016;20:1149–1151. doi: 10.1080/14728222.2016.1226284. [DOI] [PubMed] [Google Scholar]
- 23.Wagner KM, McReynolds CB, Schmidt WK, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for pain, inflammatory and neurodegenerative diseases. Pharmacol Ther. 2017;180:62–76. doi: 10.1016/j.pharmthera.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swardfager W, et al. Metabolic/inflammatory/vascular comorbidity in psychiatric disorders: Soluble epoxide hydrolase (sEH) as a possible new target. Neurosci Biobehav Rev. 2018;87:56–66. doi: 10.1016/j.neubiorev.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren Q, et al. Soluble epoxide hydrolase plays a key role in the pathogenesis of Parkinson’s disease. Proc Natl Acad Sci USA. 2018;115:E5815–E5823. doi: 10.1073/pnas.1802179115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, et al. Lipidomic profiling reveals soluble epoxide hydrolase as a therapeutic target of obesity-induced colonic inflammation. Proc Natl Acad Sci USA. 2018;115:5283–5288. doi: 10.1073/pnas.1721711115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashimoto K. Role of soluble epoxide hydrolase in metabolism of PUFAs in psychiatric and neurological disorders. Front Pharmacol. 2019;10:36. doi: 10.3389/fphar.2019.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonas RK, Montojo CA, Bearden CE. The 22q11.2 deletion syndrome as a window into complex neuropsychiatric disorders over the lifespan. Biol Psychiatry. 2014;75:351–360. doi: 10.1016/j.biopsych.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider M, et al. International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: Results from the international consortium on brain and behavior in 22q11.2 deletion syndrome. Am J Psychiatry. 2014;171:627–639. doi: 10.1176/appi.ajp.2013.13070864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose TE, et al. 1-Aryl-3-(1-acylpiperidin-4-yl)urea inhibitors of human and murine soluble epoxide hydrolase: Structure-activity relationships, pharmacokinetics, and reduction of inflammatory pain. J Med Chem. 2010;53:7067–7075. doi: 10.1021/jm100691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostermann AI, et al. Oral treatment of rodents with soluble epoxide hydrolase inhibitor 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl)urea (TPPU): Bioavailability, resulting drug levels and modulation of oxylipin pattern. Prostaglandins Other Lipid Mediat. 2015;121:131–137. doi: 10.1016/j.prostaglandins.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balan S, Toyoshima M, Yoshikawa T. Contribution of induced pluripotent stem cell technologies to the understanding of cellular phenotypes in schizophrenia. Neurobiol Dis. 2018 doi: 10.1016/j.nbd.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 33.Toyoshima M, et al. Analysis of induced pluripotent stem cells carrying 22q11.2 deletion. Transl Psychiatry. 2016;6:e934. doi: 10.1038/tp.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balan S, et al. Exon resequencing of H3K9 methyltransferase complex genes, EHMT1, EHTM2 and WIZ, in Japanese autism subjects. Mol Autism. 2014;5:49. doi: 10.1186/2040-2392-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elvevåg B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- 36.Fujita Y, Ishima T, Hashimoto K. Supplementation with D-serine prevents the onset of cognitive deficits in adult offspring after maternal immune activation. Sci Rep. 2016;6:37261. doi: 10.1038/srep37261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han M, et al. Intake of 7,8-dihydroxyflavone during juvenile and adolescent stages prevents onset of psychosis in adult offspring after maternal immune activation. Sci Rep. 2016;6:36087. doi: 10.1038/srep36087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han M, Zhang JC, Huang XF, Hashimoto K. Intake of 7,8-dihydroxyflavone from pregnancy to weaning prevents cognitive deficits in adult offspring after maternal immune activation. Eur Arch Psychiatry Clin Neurosci. 2017;267:479–483. doi: 10.1007/s00406-017-0802-1. [DOI] [PubMed] [Google Scholar]
- 39.Matsuura A, et al. Dietary glucoraphanin prevents the onset of psychosis in the adult offspring after maternal immune activation. Sci Rep. 2018;8:2158. doi: 10.1038/s41598-018-20538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guidotti A, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: A postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 41.Kimoto S, Bazmi HH, Lewis DA. Lower expression of glutamic acid decarboxylase 67 in the prefrontal cortex in schizophrenia: Contribution of altered regulation by Zif268. Am J Psychiatry. 2014;171:969–978. doi: 10.1176/appi.ajp.2014.14010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bettaieb A, et al. Soluble epoxide hydrolase deficiency or inhibition attenuates diet-induced endoplasmic reticulum stress in liver and adipose tissue. J Biol Chem. 2013;288:14189–14199. doi: 10.1074/jbc.M113.458414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inceoglu B, et al. Endoplasmic reticulum stress in the peripheral nervous system is a significant driver of neuropathic pain. Proc Natl Acad Sci USA. 2015;112:9082–9087. doi: 10.1073/pnas.1510137112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inceoglu B, Bettaieb A, Haj FG, Gomes AV, Hammock BD. Modulation of mitochondrial dysfunction and endoplasmic reticulum stress are key mechanisms for the wide-ranging actions of epoxy fatty acids and soluble epoxide hydrolase inhibitors. Prostaglandins Other Lipid Mediat. 2017;133:68–78. doi: 10.1016/j.prostaglandins.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seubert JM, Zeldin DC, Nithipatikom K, Gross GJ. Role of epoxyeicosatrienoic acids in protecting the myocardium following ischemia/reperfusion injury. Prostaglandins Other Lipid Mediat. 2007;82:50–59. doi: 10.1016/j.prostaglandins.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell WB. New role for epoxyeicosatrienoic acids as anti-inflammatory mediators. Trends Pharmacol Sci. 2000;21:125–127. doi: 10.1016/s0165-6147(00)01472-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.