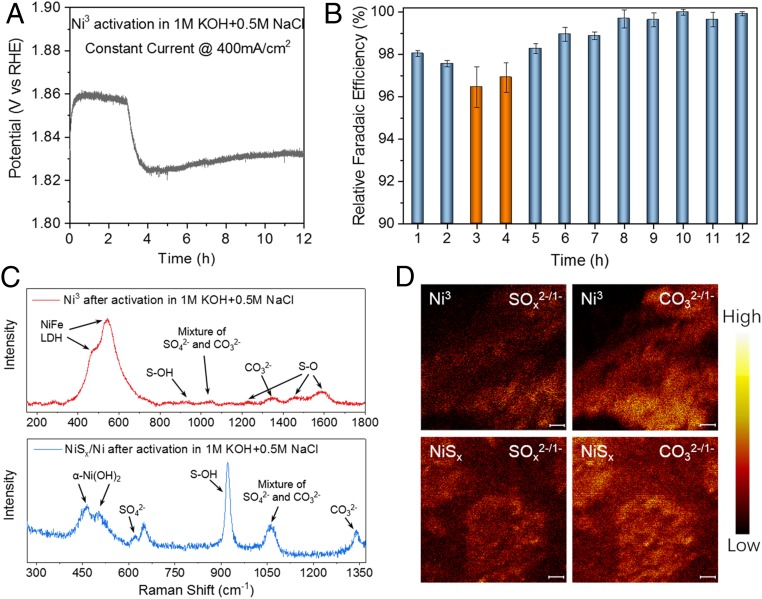
Fig. 4.
Cation selective layer generation during activation in salty electrolyte. (A) Three-electrode OER constant current activation of Ni3 in 1 M KOH + 0.5 M NaCl, resistance 1.4 ± 0.05 ohm, electrode area 0.5 cm2. The decrease in voltage that occurred between 3 and 4 h was due to the etching-passivation process. Note that Ni3 was first activated in 1 M KOH under 400 mA/cm2 for 12 h before activation in 1 M KOH + 0.5 M NaCl. (B) OER R_FE plots for O2 production taken during A. Decrease in voltage at 3 to 4 h corresponds to a small decrease in R_FE. Error bars were obtained by three parallel tests. (C) Raman spectra of Ni3 and NiSx/Ni after 12-h activation in 1 M KOH + 0.5 M NaCl, suggesting polyatomic anion intercalated LDH phase and formation of sulfate species at the LDH/NiSx interface. (D) TOF-SIMS mapping of SOx2-/1- and CO32-/1- fragments from a Ni3 and NiSx/Ni electrode surface after activation in 1 M KOH + 0.5 M NaCl at 400 mA/cm2. Negative TOF-SIMS counts were collected from m/z = 96/48/80/40 (SO4−/SO42-/SO3−/SO32-) and 60/30 (CO3−/CO32-) after Ar plasma milling for 5 to 15 min to clean the surface adsorbed electrolytes. (Scale bars: 10 μm.)