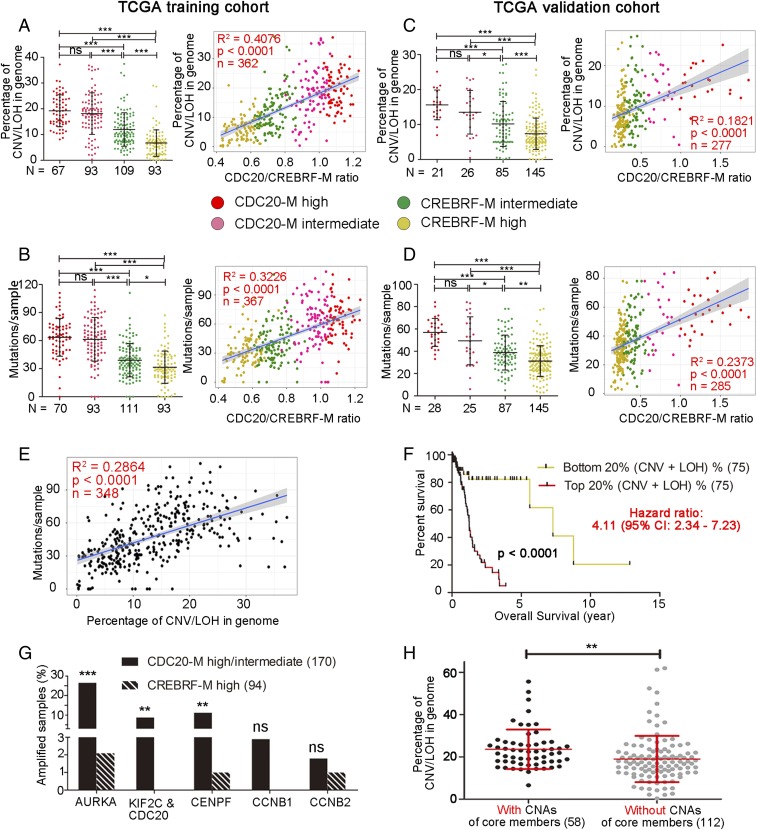
Fig. 3.
Elevated CDC20-M signature marks extensive chromosome abnormalities and mutation burden in glioma. (A and B, Left) The extent of chromosomal abnormalities (A) or mutation burden (B) in the CDC20-M–defined glioma subgroups in the TCGA training cohort. ***P < 0.001; ns: P > 0.05 as analyzed in one-way ANOVA test. (Right) Linear regression analysis between the ratio of CDC20-M/CREBRF-M and the extent of chromosomal abnormalities or mutation burden in glioma samples. The same color codes were used in all panels. Regression coefficient (R2), P value, and number of samples (n) are indicated. (C and D, Left) The extent of chromosomal abnormalities (C) or mutation burden (D) in the SSP-defined CDC20-M glioma subgroups in the TCGA validation cohort. ***P < 0.001; **P < 0.01; *P < 0.05; ns: P > 0.05 as analyzed in one-way ANOVA test. (Right) Linear regression analysis between the ratio of CDC20-M/CREBRF-M and the extent of chromosomal abnormalities or mutation burden in glioma samples. (E) Linear regression analysis between the extent of chromosomal abnormalities and mutation burdens per glioma sample in the TCGA training cohort. Regression coefficient (R2), P value, and number of samples (n) are indicated. (F) Survival comparison between the patients with gliomas with the top 20% most severe chromosomal abnormalities and the patients with gliomas with the lowest 20% chromosomal abnormalities in the TCGA training cohort. (G) Percentage of glioma samples with amplifications in the six core members of CDC20-M in the TCGA training cohort. Copy numbers were analyzed using GISTIC 2.0. ***P < 0.001, **P < 0.01, ns: P > 0.05. Fisher’s exact test was applied to compare CDC20-M–high/intermediate with CREBRF-M–high samples. (H) Comparison of the extent of chromosomal abnormalities between samples harboring amplifications of any of the core members (AURKA, CDC20, KIF2C, CENPF) and samples without amplification of these core members in the glioma with high or intermediate CDC20-M expression. **P < 0.01, unpaired two-sided Student’s t test.