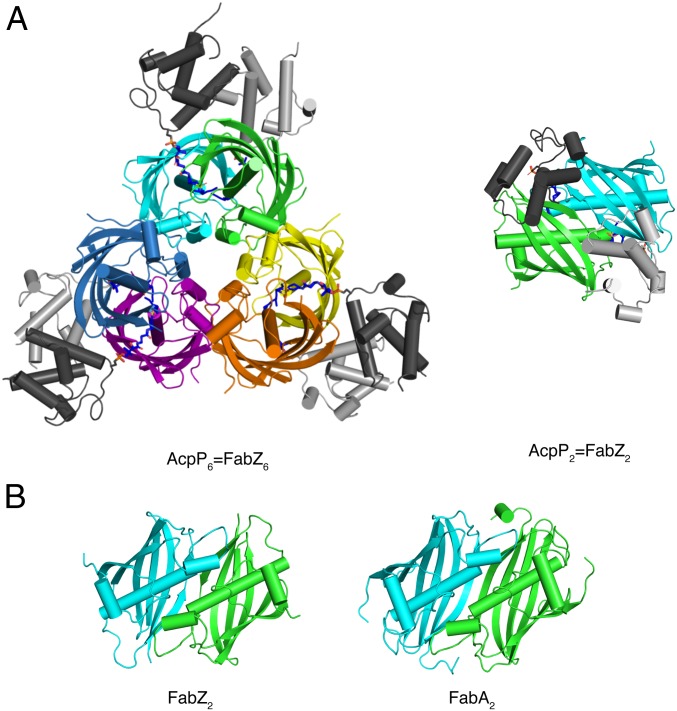
Fig. 3.
Structure of AcpP=FabZ. (A) E. coli FabZ hexamer with six AcpPs. The FabZ hexamer is composed of three dimers (cyan and green, yellow and orange, purple and blue). (Left) The six AcpPs are at the periphery (dark and light gray), each cross-linked to a FabZ subunit. (Right) A “top-down” view of the cyan/green AcpP=FabZ dimer shows that the two bound AcpPs do not contact one another. The DH6 cross-linkers are rendered as sticks in atomic coloring (blue N, red O, yellow S, and dark or light gray C according to the tethered ACP). (B) Comparison of a FabZ dimer from the hexamer in A (Left) and E. coli FabA (Right), which is a dimer. FabZ and FabA have identical folds and dimer interfaces; FabZ is a trimer of FabA-like dimers, whereas FabA is a dimer. The FabZ cyan/green dimer in B is rotated 90° from the right view in A.