Abstract
Background
Our aim was to assess whether the use of cycloserine (CS) would bring additional benefit for multidrug-resistant tuberculosis (MDR-TB) patients, and to estimate the incidence and associated risk factors of adverse drug reactions (ADRs) from CS.
Patients and methods
In this study, we retrospectively reviewed the clinical outcomes and ADRs of MDR-TB patients treated with CS containing regimens between January 2012 and June 2015 in China.
Results
A total of 623 MDR-TB cases enrolled in this study received regimens containing CS. Of these cases, in 411 of the patients 374 (66.0%) were “cured” and 37 (5.9%) “complete treatment” by the end of the study. The elderly, patients with prolonged previous exposure to and history of anti-TB drugs, and pre-existing co-morbidity were more likely to be associated with adverse outcomes of MDR-TB patients (P<0.05). Hyperuricemia (22.8%, 142/623) was the most frequently observed ADR among these cases, while the most noted ADRs associated with the administration of CS was psychiatric symptoms, accounting for 4.3% (27/623) of study population. Nineteen (70.4%) out of 27 cases with psychiatric symptoms occurred before the 6-month timepoint, and were notably, the highest proportion of serious adverse, 29.6% (8/27) of which were noted after discontinuation of CS.
Conclusion
Our study demonstrates that a CS-containing regimen achieved a highly successful outcome in the treatment of MDR-TB and promising tolerance in Chinese population. The potential emergence of serious psychiatric symptoms highlights that patients need to be closely monitored for these conditions during treatment that includes CS.
Keywords: multidrug-resistant tuberculosis, cycloserine, treatment, China, adverse events
Introduction
Multidrug-resistant tuberculosis (MDR-TB), caused by bacteria that are resistant to at least isoniazid and rifampicin, are major obstacles in global TB control efforts.1,2 The WHO estimated that 490,000 new cases of MDR-TB developed worldwide in 2016, and almost half of all MDR-TB cases occurred in India and China.3 Management of MDR-TB is challenging because it requires prolonged medical therapy with conventional second-line drugs, which are more expensive and toxic, but less efficacious in comparison to the first-line drugs.4 As a consequence, MDR-TB cases have to face high rates of failure, loss to follow-up, relapse and death. Globally, only 52% of MDR-TB patients successfully completed treatment in 2013,5 highlighting the urgent need for more effective anti-TB agents and optimal use of currently available drugs against this intractable form of TB.
Cycloserine (CS) is a broad-spectrum antibiotic with moderate in vitro bacterial and clinical activity against Mycobacterium tuberculosis (MTB).6,7 CS is categorized as a second-line Group C oral bacteriostatic drug by the WHO.8 Notably, this drug does not show any cross-resistance with other anti-TB drugs due to its unique mode of action.7,9 Hence, CS is widely used for the treatment of drug-resistant TB, especially MDR-TB. As one of the most important agents for treating MDR-TB, the primary concern affecting the use of CS is its known adverse drug reactions (ADRs).7 Of various ADRs associated with the administration of CS, psychiatric symptoms are the most frequently reported ADRs, including headaches, depression and mental disturbances.10 Although these psychiatric ADRs do not result in significant morbidity, they no doubtly compromise adherence to treatment and prevention of drug resistance.11
China had the second highest MDR-TB incidence worldwide, with estimated 58,000 incident MDR-TB cases in 2016.3 On the basis of the recent drug-resistant survey, 5.7% of new cases and 25.6% of retreatment cases in China had MDR-TB.12 Additionally, only 49% of MDR-TB patients were successfully treated in China in a national retrospective report.13 Despite the recommendation of CS for the treatment of drug-resistant TB by the WHO, the National TB Program of China has only recently endorsed the use of CS in clinical practices due to toxicity concerns.14 Hence, the data regarding the efficacy and safety of CS are limited in China. In this study, we retrospectively reviewed the clinical outcomes and ADRs of MDR-TB patients treated with CS containing regimens in China. Our aim was to assess whether the use of CS would bring additional benefit for MDR-TB patient, and to estimate the incidence and associated risk factors of ADRs from CS.
Patients and methods
Ethics statement
The study was approved by the Ethics Committee of Chinese Center for Disease Control and Prevention. Written informed consent was obtained from each patient prior to enrolment in this study.
Patients
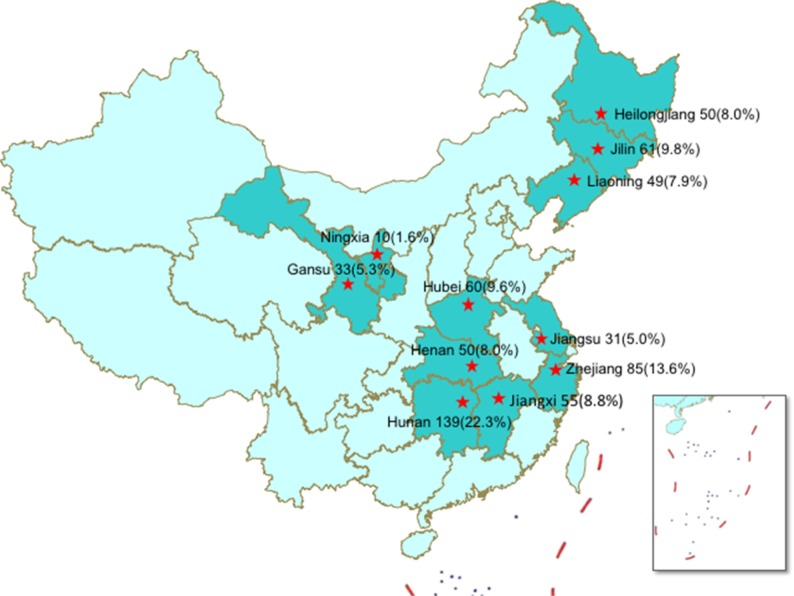
We carried out a retrospective study in eleven TB hospitals supported by a Global Fund for the treatment of MDR-TB patients, including Heilongjiang, Jilin, Liaoning, Henan, Hunan, Hubei, Jiangxi, Gansu, Ningxia, Jiangsu and Zhejiang between January 2012 and June 2015 (Figure 1). The patients enrolled in this study met the following criteria: 1) MDR-TB patients diagnosed by conventional drug susceptibility testing; 2) patients aged 18–70 years; 3) patients receiving the regimen containing CS. All XDR-TB patients, defined as MDR-TB plus resistant to any fluoroquinolone and any second-line injectable drug, were excluded from this study. The demographic and clinical characteristics were collected from hospital medical records, including sex, age, BMI, treatment history, treatment duration of previously treated patients and comorbidity.
Figure 1.
Distribution map of multidrug-resistant tuberculosis patients.
Clinical treatment and monitoring of adverse events
A standardized treatment regimen for the treatment of MDR-TB patients consisted of a 6-month intensive phase, followed by an 18-month continuation phase: 6 Z+Km (Am, Cm)+ Lfx (Mfx)+ Pto + Cs/18 Z+Lfx (Mfx)+ Pto + Cs (Am, amikacin; Cm, capreomycin; Cs, cycloserine; E, ethambutol; Lfx, levofloxacin; Mfx, moxifloxacin; PAS, p-aminosalicylic acid; Pto, protionamide; Z, pyrazinamide). To evaluate the clinical outcomes of MDR-TB cases, routine follow-up examinations and laboratory tests were performed every month during the 6-month intensive phase, and every 2 months during the 18-month continuation phase. Treatment outcomes were assessed by the end of MDR-TB treatment to classify the patients as cured, complete, death during treatment, failure, or loss to follow-up according to standard WHO definitions. Cure was defined by at least three consecutive negative cultures and no positive culture during the last 18 months of treatment. Treatment completion was defined by bacteriological conversion through the end of treatment but fewer than three consecutive negative culture. Death was defined as death for any reason during the course of MDR-TB treatment. Default was defined as treatment interruption for two or more consecutive months for any reason without medical approval. Treatment failure was defined as persistence of two or more positive cultures of the five cultures recorded in the final 12 months, persistence of one or more positive cultures in the final 3 months, or early treatment termination because of poor clinical or radiological response or adverse events. Cured and treatment completed were categorized as successful outcomes, while adverse outcome included any death, default, and treatment failure.14
We monitored the safety and tolerability of treatment through patient self-reporting, blood count, ALT level, AST level and uric acid examination. Hepatic damage was defined as the elevation of serum transaminases to at least three times the normal values in the presence of gastrointestinal symptoms, or evaluation of serum transaminases to at least five times normal values without symptoms.15 Renal damage was defined as the elevation of creatinine to at least 1.3 times normal values. Hyperuricemia was defined as a serum uric acid level greater than 7.0 mg/dL.15 Psychiatric disorder was defined as the presence of insomnia, anxiety, depression, mania and psychosis. The severity of grading of psychiatric disorder was performed according to a recent report.16 Serious adverse event was defined as any adverse reaction resulting in discontinuation of one or more anti-TB drugs.
Statistical analysis
Categorical data were analyzed using the Pearson Chi-squared test or Fisher’s exact test. A bivariate logistic regression model was constructed to assess the factors associated with the presence of adverse events. Variables with P<0.1 were considered for inclusion in multivariable modeling. If P<0.05, differences were declared as statistically significant. All calculations were completed in SPSS 17.0 (SPSS Inc., Chicago, IL, USA USA).
Results
Demographic and clinical characteristics
A total of 623 individuals infected with MDR-TB were enrolled in this study. The mean age at diagnosis was 42.8 years, ranging in age from 18 to 70 years. 71.8% (447/623) of these cases were male, and the remaining 28.2% (176/623) were female. Four hundred and ninety-eight (79.9%) had previously been treated for TB, while all of them were never exposed to CS during the prior treatment. Of these previously treated cases, the previous treatment duration ranged from 1 to 40 months, with a mean duration of 17.8 months. Diabetes was the most frequent comorbidity (96), followed by hypertension (9) and COPD (4). Of the MTB strains isolated from MDR-TB patients, 161 (25.8%) were resistant to ethambutol, 124 (19.9%) to ofloxacin, and 85 (13.6%) to amikacin (Table 1).
Table 1.
Demographic and clinical characteristics of MDR-TB patients
| Characteristics | Experimental group (N=623) |
|---|---|
|
| |
| Age, years, mean (range) | 42.8 (18−70) |
| Male sex, No. (%) | 447 (71.8) |
| BMI kg/m2, mean (range) | 19.9 (12.57−32.45) |
| Treatment history, No. (%) | |
| New cases | 125 (20.1) |
| Previously treated | 498 (79.9) |
| Treatment duration of previously treated patients (months) | 17.8 (1−40) |
| Co-morbidity, No. (%) | |
| Diabetes | 96 (15.4) |
| Hypertension | 9 (1.4) |
| COPD | 4 (0.6) |
| Cardiopathy | 3 (0.5) |
| Others | 28 (4.5) |
| Course of disease, No. (%) | |
| ≤1 year before | 190 (30.5) |
| >1 year to 3 years before randomization | 155 (24.9) |
| >3 years before randomization | 278 (44.6) |
| Susceptibility test, resistant, No. (%) | |
| Isoniazid | 623 (100.0) |
| Rifampicin | 623 (100.0) |
| Streptomycin | 577 (92.6) |
| Ethambutol | 161 (25.8) |
| Ofloxacin | 124 (19.9) |
| Kanamycin | 85 (13.6) |
Abbreviation: MDR-TB, multidrug-resistant tuberculosis.
Treatment outcome
Of the 623 MDR-TB patients receiving regimens with CS, 411 (66.0%, 411/623) met the definition of “cure” and 37 (5.9%, 37/623) “complete treatment” for a total of 448 (71.9%) who had favorable outcomes, including 93 cases from new case group. In contrast, 68 (10.9%) died by study completion, 11 (1.8%) failed treatment, and 96 (15.4%) were loss to follow-up during treatment (Table 2). We further analysed the factors associated with adverse treatment outcomes. As summarized in Table 3, patients who were aged ≥65 years were significantly more likely to experience treatment failure compared with patients who were <25 years old (adjusted odds ratio [aOR]: 2.755, 95% CI: 1.119–6.784). In addition, the duration of previous exposure to anti-TB regimens was a significantly predictor of adverse outcomes. Compared with the patients with ≤1-year previous exposure, the patients with 1–3 years (P<0.001) or >3 years previous exposure (P=0.001) were significantly more likely to have adverse treatment outcomes. A significantly higher percentage of poor outcome was observed in MDR-TB cases with comorbidities (40.7%) when compared to those without comorbidities (24.4%, P=0.001), demonstrating that the underlying comorbidity was independently associated with greater hazard of poor clinical outcome.
Table 2.
Clinical outcome of patients
| Treatment outcome | No. of patients (%) |
|---|---|
|
| |
| Favorable outcome | |
| Cure | 411 (66.0) |
| Treatment completion | 37(5.9) |
| Adverse outcome | |
| Failure | 68(10.9) |
| Death | 11 (1.8) |
| Default | 96 (15.4) |
Table 3.
Factors associated with adverse outcomes among MDR-TB patients
| Characteristics | Favorable outcome (N=448) | Adverse outcome (N=175) | Odds ratios (95% CI) | P-value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| No. | % | No. | % | |||
|
| ||||||
| Age (years) | ||||||
| <25 | 62 | 76.5 | 19 | 23.5 | Ref | |
| 25–44 | 174 | 73.7 | 62 | 26.3 | 2.104 (0.753–5.880) | 0.156 |
| 45–64 | 197 | 71.4 | 79 | 28.6 | 2.465 (0.980–6.199) | 0.055 |
| >65 | 13 | 50.0 | 13 | 50.0 | 2.755 (1.119–6.784) | 0.028 |
| Sex | ||||||
| Male | 301 | 68.7 | 137 | 31.3 | Ref | |
| Female | 135 | 78.0 | 38 | 22.0 | 1.495 (0.933–2.394) | 0.095 |
| BMI | ||||||
| <18.5 | 114 | 67.1 | 56 | 32.9 | Ref | |
| 18.5–23.9 | 230 | 71.0 | 94 | 29.0 | 0.546 (0.239–1.248) | 0.152 |
| >24 | 32 | 72.7 | 12 | 27.3 | 0.709 (0.328–1.533) | 0.382 |
| Course of disease | ||||||
| ≤1 year before | 173 | 74.9 | 58 | 25.1 | Ref | |
| >1 year to 3 years before randomization | 103 | 73.0 | 38 | 27.0 | 0.748 (0.414–1.350) | 0.335 |
| >3 years before randomization | 172 | 68.5 | 79 | 31.5 | 0.831 (0.484–1.426) | 0.501 |
| Treatment history | ||||||
| New cases | 86 | 74.8 | 29 | 25.2 | Ref | |
| Previously treated | 362 | 71.3 | 146 | 28.7 | 0.737 (0.408–1.329) | 0.310 |
| Treatment duration of patients (months) | ||||||
| ≤1 year before | 244 | 76.2 | 76 | 23.8 | Ref | |
| >1 year to 3 years before randomization | 173 | 71.8 | 68 | 28.2 | 3.907 (1.949–7.832) | <0.001 |
| >3 years before randomization | 31 | 50.0 | 31 | 50.0 | 3.103 (1.621–5.940) | 0.001 |
| Adverse effect | ||||||
| Yes | 225 | 72.6 | 85 | 27.4 | Ref | |
| No | 223 | 71.2 | 90 | 28.8 | 1.091 (0.734–1.621) | 0.666 |
| Co-morbidity | ||||||
| Yes | 83 | 59.3 | 57 | 40.7 | Ref | |
| No | 365 | 75.6 | 118 | 24.4 | 2.262 (1.397–3.662) | 0.001 |
Abbreviations: Abbreviation: MDR-TB, multidrug-resistant tuberculosis.
Safety
A total of 445 adverse events occurred in 316 patients reported during MDR-TB treatment, 45 (10.1%) of which were serious adverse events. Of 316 patients experiencing adverse events, 77 (24.4%) and 239 (75.6%) were from new cases and previously treated case group, respectively. As shown in Table 4, the most common adverse event was hyperuricemia (142), followed by hepatotoxicity (117), renal damage (37) and hearing loss (36). In addition, 27 patients (4.3%) developed psychiatric symptoms attributed to CS. Of the 45 serious adverse events, 15 (23.8%) had hepatic damage, 8 (17.8%) had psychiatric symptoms, and 6 (13.3%) had gastrointestinal reaction. Nineteen of the treated patients (70.4%) experienced psychiatric symptoms of varying severity within the first 6 months of treatment, and the other eight patients (29.6%) had psychiatric symptoms during the subsequent 7 months (Figure 2).
Table 4.
Adverse events during 24-month treatment among patients enrolled in the study
| Classification | No. of reported ADRs (%)
|
Prevalence of serious ADR among patients experiencing this type of ADR (%) | |
|---|---|---|---|
| ADR (N=445) | Severe ADR (N=45) | ||
|
| |||
| Hyperuricemia | 142 (31.9) | 4 (8.9) | 2.8 |
| Hepatotoxicity | 117 (26.3) | 15 (33.3) | 12.8 |
| Renal damage | 37 (8.3) | 1 (2.2) | 2.7 |
| Tinnitus or hearing loss | 36 (8.1) | 2 (4.4) | 5.6 |
| Psychiatric symptoms | 27 (6.1) | 8 (17.8) | 29.6 |
| Gastrointestinal reaction | 26 (5.8) | 6 (13.3) | 23.1 |
| Others | 60 (13.5) | 9 (20.0) | 15.0 |
Abbreviation: ADR, adverse drug reaction.
Figure 2.
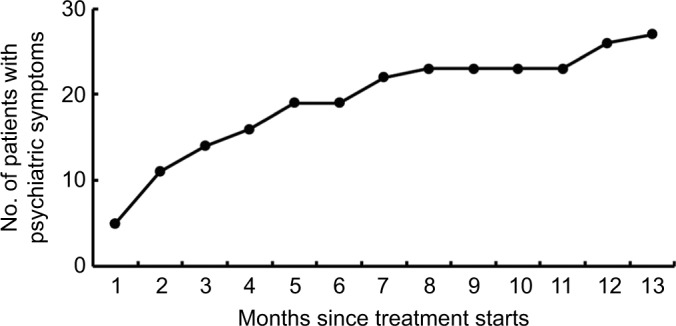
Cumulative number of patients with psychiatric symptoms during anti-tuberculosis treatment course.
We further analyzed the rate of patients with serious adverse events according to different adverse event types. The highest proportion of serious adverse events observed was psychiatric symptom, 29.6% (8/27) of whom were noted with discontinuation of CS. In contrast, despite 142 patients suffering from hyperuricemia, only 4 (2.8%) developed serious adverse events (Table 4). Statistical analysis was also performed to determine the risk factors contributing to the occurrence of psychiatric symptom due to CS, while we found no significance in the rate of psychiatric symptom among various characteristic groups (P>0.05) (Table 5).
Table 5.
Risk factors associated with psychiatric symptoms
| Characteristics | Psychiatric symptoms
|
OR (95% CI) | P-value | |
|---|---|---|---|---|
| No | Yes | |||
|
| ||||
| Age (years) | ||||
| <25 | 24 | 0 | ref | |
| 25–44 | 210 | 10 | 1.035 (1.013–1.058) | 0.998 |
| 45–64 | 293 | 15 | 1.042 (1.020–1.063) | 0.329 |
| >65 | 69 | 2 | 1.036 (0.987–1.087) | 0.338 |
| Sex | ||||
| Male | 418 | 20 | ref | |
| Female | 178 | 7 | 1.047 (0.436–2.512) | 0.949 |
| BMI | ||||
| <18.5 | 191 | 10 | ref | |
| 18.5–23.9 | 355 | 14 | 1.357 (0.594–3.099) | 0.707 |
| >24 | 50 | 3 | 0.773 (0.206–2.899) | 0.574 |
| Co-morbidity | ||||
| Yes | 132 | 7 | ref | |
| No | 464 | 20 | 0.447 (0.187–1.069) | 0.538 |
| Outcome of treatment | ||||
| Favorable outcome | 432 | 16 | ref | |
| Adverse outcome | 164 | 11 | 0.565 (0.256–1.244) | 0.084 |
Discussion
CS was initially used for the treatment of complicated or resistant TB cases half a century ago.6 Despite producing satisfactory outcomes in a majority of patients in whom other regimens failed, the lack of definitive outcome trials limits the use of CS in clinical practice. In this study, the clinical efficacy and safety of CS in patients infected with MDR-TB was analysed among 623 patients from 11 clinical centers in China. Briefly, 72% of patients had treatment success under treatment with CS-containing regimen, which was significantly higher than that of earlier cohorts of MDR-TB patients in China, indicating that CS promoted culture conversation in many MDR-TB patients. We hypothesize that several reasons may be contributing to the improved efficacy for MDR-TB patients in our observation. Our study was part of Global Fund Project, in which the additional effort had been taken in the patient management. Consequently, the improved medication compliance of patients undoubtedly translates into better clinical outcomes. Additionally, all patients enrolled in this study had never been exposed to CS. Hence, the low potential of CS resistance may be another important reason for the more successful outcomes. Third, as an analog of D-alanine, CS inhibits L-alanine racemase and dipeptidyl synthetase, thus blocking the cell wall bacterial cell wall synthesis.17 An early study showed that treatment of the bacteria with CS caused about 90% inhibition of cell wall synthesis.18 Hence, we speculate that the CS in the regimen could impair the cell wall barrier of tubercle bacillus, and increase the penetration of other drugs into the cell, thus improving the efficacy of CS-containing regimens against MDR-TB. Further experimental evidence is necessary to elucidate the potential synergistic effect between CS and other drugs in the treatment of MDR-TB pulmonary disease.
Older age is a well-known risk factor for treatment failure of patients with TB.19 In consistency with to previous studies, patients who were aged ≥65 years were more likely to have poor clinical outcomes.13,19 It is possible that the reduced immunity in this population may favor the progression of the disease and limit the efficacy of anti-TB drugs, thereby increasing the risk of poor outcome.20 It is also possible that the elderly patients have significantly more problems understanding their medical regimens than younger patients, possibly contributing to the high incidence of default rate in the elderly group.21 Poor adherence linked with adverse treatment outcomes emphasized the importance of directly observed therapy in the treatment of elderly MDR-TB patients.
In addition, the underlying co-morbidities were found to be independently associated with adverse outcomes of MDR-TB patients. In our patients, diabetes was the most common form of co-morbidity, accounting for 68.6% of patients with various comorbid conditions. Numerous investigations have been undertaken that suggest a strong link between diabetes and increased risk of poor clinical outcomes among TB patients,22,23 which would be contributed to impaired cell-medicated immunity due to diabetes. Given the growing epidemic of diabetes mellitus in China, the concurrence of both diseases potentially carries a risk of national wide spreading, with serious implications for TB control and the achievement of the WHO post-2015 END TB strategy goal in the future.
Psychiatric symptoms are the most predominant adverse events associated with the use of CS. A recent meta-analysis by Hwang et al revealed that the pooled estimate for the frequency of psychiatric ADRs from CS was 5.7%,7 which is similar to our observation that 4.3% of patients experienced psychiatric symptoms due to CS. Despite exhibiting promising safety, we found that the administration of CS produces a higher proportion of serious ADRs resulting in discontinuation of CS. In addition, low plasma concentration is another important concern for the clinical application of CS.24,25 Although the increased dosage of CS could overcome this obstacle, it may also pose a higher safety risk. Our results indicate that CS should be considered as one of the essential components of MDR-TB regimens, while the monitoring of psychiatric symptoms is necessary to prevent the presence of serious ADRs.
Limitations
Our analyses are subject to several limitations. Firstly, this retrospective study was carried out in 11 hospitals rather all the pilots supported by Global Fund. This sampling bias may be a source of inaccuracy in the interpretation of results. Secondly, psychiatric disorders are cultural taboos in China, resulting in the historically undercounted status of such conditions. In view of these circumstances, the estimated rate of psychiatric symptoms due to the use of CS may be underestimated. Finally, the correlation between drug susceptibility results of testing in vitro and therapeutic outcome in vivo for CS was not assessed because of inaccessibility of in vitro susceptibility to CS in these pilots. Further studies are warranted to evaluate the relationship between CS resistance and patient outcomes in the clinical trials.
Conclusion
Our data demonstrates that CS provides satisfactory efficacy against MDR-TB and promising tolerance in Chinese population. Elderly patients, prolonged previous exposure history to anti-TB drugs, and pre-existing co-morbidity are more likely to be associated with adverse outcomes in MDR-TB patients. The potential emergence of serious psychiatric symptoms highlights that patients need to be closely monitored for these conditions during treatment that includes CS.
Acknowledgments
We express our thanks to local staff for their time and effort in data collection and patient follow-up. This work was supported by the Ministry of Science and Technology of the People’s Republic of China (2017ZX09304009).
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gandhi NR, Nunn P, Dheda K, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 2010;375(9728):1830–1843. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- 2.Marks SM, Flood J, Seaworth B, et al. Treatment practices, outcomes, and costs of multidrug-resistant and extensively drug-resistant tuberculosis, United States, 2005-2007. Emerg Infect Dis. 2014;20(5):812–821. doi: 10.3201/eid2005.131037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Global Tuberculosis Report 2017. Geneva: World Health Organization; 2017. [Google Scholar]
- 4.Yu M-C, Chiang C-Y, Lee J-J, et al. Treatment outcomes of multidrug-resistant tuberculosis in Taiwan: tackling loss to follow-up. Clin Infect Dis. 2018;67(2):202–210. doi: 10.1093/cid/ciy066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . Global Tuberculosis Report 2014. Geneva: World Health Organization; [Google Scholar]
- 6.Epstein IG, Nair KG, Boyd LJ. Cycloserine, a new antibiotic, in the treatment of human pulmonary tuberculosis: a preliminary report. Antibiotic Med Clin Ther. 1955;1(2):80–93. [PubMed] [Google Scholar]
- 7.Hwang TJ, Wares DF, Jafarov A, Jakubowiak W, Nunn P, Keshavjee S. Safety of cycloserine and terizidone for the treatment of drug-resistant tuberculosis: a meta-analysis. Int J Tuberc Lung Dis. 2013;17(10):1257–1266. doi: 10.5588/ijtld.12.0863. [DOI] [PubMed] [Google Scholar]
- 8.Who Treatment Guidelines for Drug-Resistant Tuberculosis, 2016 Update. Geneva: World Health Organization; 2016. WHO guidelines approved by the guidelines review Committee. [Google Scholar]
- 9.Lambert MP, Neuhaus FC. Mechanism of D-cycloserine action: alanine racemase from Escherichia coli W. J Bacteriol. 1972;110(3):978–987. doi: 10.1128/jb.110.3.978-987.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yew WW, Wong CF, Wong PC, Lee J, Chau CH. Adverse neurological reactions in patients with multidrug-resistant pulmonary tuberculosis after coadministration of cycloserine and ofloxacin. Clin Infect Dis. 1993;17(2):288–289. doi: 10.1093/clinids/17.2.288. [DOI] [PubMed] [Google Scholar]
- 11.Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003;167(11):1472–1477. doi: 10.1164/rccm.200206-626OC. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Xu S, Wang L, et al. National survey of drug-resistant tuberculosis in China. N Engl J Med. 2012;366(23):2161–2170. doi: 10.1056/NEJMoa1108789. [DOI] [PubMed] [Google Scholar]
- 13.Xu C, Pang Y, Li R, et al. Clinical outcome of multidrug-resistant tuberculosis patients receiving standardized second-line treatment regimen in China. J Infect. 2018;76(4):348–353. doi: 10.1016/j.jinf.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Laserson KF, Thorpe LE, Leimane V, et al. Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2005;9(6):640–645. [PubMed] [Google Scholar]
- 15.Wang Q, Pang Y, Jing W, et al. Clofazimine for treatment of extensively drug-resistant pulmonary tuberculosis in China. Antimicrob Agents Chemother. 2018;62(4):e02149–17. doi: 10.1128/AAC.02149-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Division of AIDS, National Institute of allergy and infectious diseases National Institutes of health, US department of health and Human services Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events: corrected version 2.1. 2017. [Accessed March 20, 2019]. Available from: https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcor-rectedv21.pdf.
- 17.Brennan PJ, Crick DC. The cell-wall core of Mycobacterium tuberculosis in the context of drug discovery. Curr Top Med Chem. 2007;7(5):475–488. doi: 10.2174/156802607780059763. [DOI] [PubMed] [Google Scholar]
- 18.Horne D, Tomasz A. Tolerant response of Streptococcus sanguis to beta-lactams and other cell wall inhibitors. Antimicrob Agents Chemother. 1977;11(5):888–896. doi: 10.1128/aac.11.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aung KJM, van Deun A, Declercq E, et al. Successful ‘9-month Bangladesh regimen’ for multidrug-resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis. 2014;18(10):1180–1187. doi: 10.5588/ijtld.14.0100. [DOI] [PubMed] [Google Scholar]
- 20.Rajagopalan S. Tuberculosis and aging: a global health problem. Clin Infect Dis. 2001;33(7):1034–1039. doi: 10.1086/322671. [DOI] [PubMed] [Google Scholar]
- 21.Martin LR, Williams SL, Haskard KB, Dimatteo MR. The challenge of patient adherence. Ther Clin Risk Manag. 2005;1(3):189–199. [PMC free article] [PubMed] [Google Scholar]
- 22.Baker MA, Harries AD, Jeon CY, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Workneh MH, Bjune GA, Yimer SA. Prevalence and associated factors of tuberculosis and diabetes mellitus comorbidity: a systematic review. PLoS One. 2017;12(4):e0175925. doi: 10.1371/journal.pone.0175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu H, Guo S-C, Liu Z-Q, et al. Therapeutic drug monitoring of cycloserine and linezolid during anti-tuberculosis treatment in Beijing, China. Int J Tuberc Lung Dis. 2018;22(8):931–936. doi: 10.5588/ijtld.17.0648. [DOI] [PubMed] [Google Scholar]
- 25.Yu X, Zeng X, Shi W, et al. Validation of cycloserine efficacy in treatment of multidrug-resistant and extensively drug-resistant tuberculosis in Beijing, China. Antimicrob Agents Chemother. 2018;62(3) doi: 10.1128/AAC.01824-17. e01824–17pii. [DOI] [PMC free article] [PubMed] [Google Scholar]