Figure 2. Evaluation of enzymatic activity for the wild‐type and mutated promiscuous enzyme, YihS.
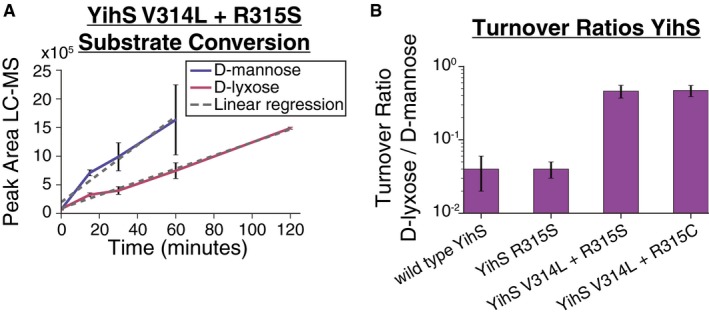
- YihS V314L + R315S mutant enzyme activity on D‐mannose and D‐lyxose. LC‐MS was used to analyze YihS activity at saturating substrate concentrations to compare turnover rates on each substrate. Product formation was followed over time at a constant enzyme concentration. Turnover rates were calculated using linear regression (n = 3 replicates for each enzyme, Dataset EV4). The error bars represent standard deviation (n = 3) of the peak area.
- Turnover ratios of substrate conversion of D‐lyxose/D‐mannose are shown for the wild‐type YihS and mutant YihS enzymes. A ratio < 1 indicates a higher turnover rate on D‐mannose compared to D‐lyxose. Error bars represent standard error (n = 3) calculated from the linear regression analysis.
