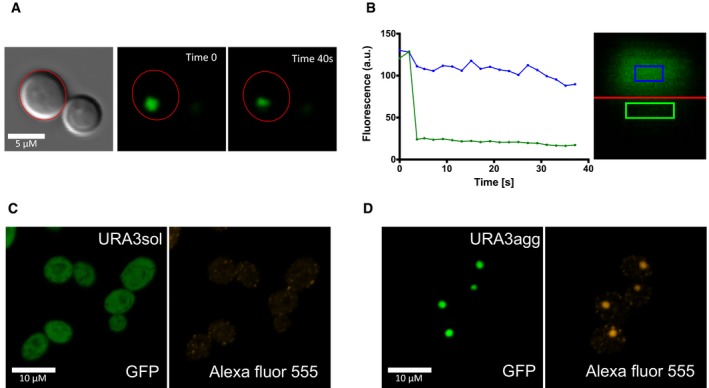
We tested the consistency of the URA3
agg foci by photobleaching one half of the deposit (green line and green square) and monitoring the protein diffusion from the other half (blue line and blue square) with confocal microscopy. The assay was monitored for 40 seconds and shows no fluorescence loss or gain in any of the sides. This indicates that these foci are very dense, very much like an insoluble protein deposit (IPOD; Kaganovich
et al,
2008).
-
A
Foci before (Time 0 s) and after (Time 40 s) photobleaching.
-
B
Graph showing the fluorescence changes along time in each side of the foci.
-
C, D
We also tested the conformation of the protein enclosed in the URA3agg foci by analyzing the binding of anti‐oligomer antibodies (rabbit anti‐oligomer (A11) AHB0052). As a secondary antibody, we used goat anti‐rabbit IgG H&L tagged with an Alexa Fluor® 555 (ab150078). We obtained a bright fluorescence signal that colocalizes with the foci's GFP fluorescence, indicating that the deposits are rich in oligomeric structures. As a control, we also incubated URA3sol with these antibodies obtaining few faint Alexa Fluor foci at the cytoplasm, indicating the absence or very low presence of oligomers.