Figure 4. Utilization of graphene-Au hybrid NEAs as an electrochemical sensing platform for in-situ monitoring of osteogenic differentiation of hMSCs.
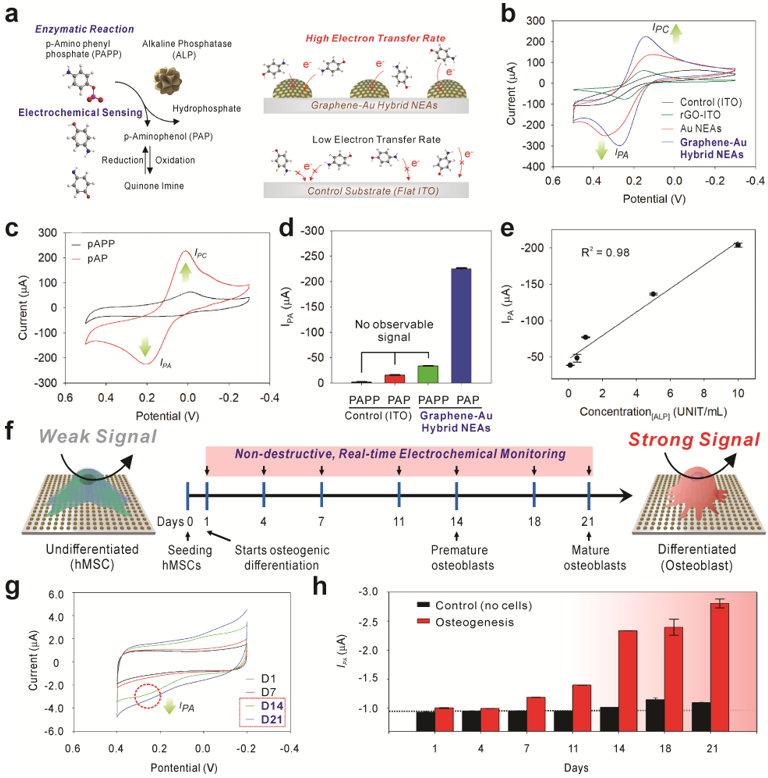
a) Schematic illustration of an enzymatic reaction and electrochemical sensing mechanism of alkaline phosphatase (ALP) and improved electron transfer kinetic based on the 3D surface in graphene-Au hybrid NEAs compared to 2D flat ITO surface. b) Cyclic voltammogram of 1 mM of [Fe(CN)6]4− dissolved in DPBS obtained at a 50 mV s−1 scan rate using a bare ITO substrate, rGO coated ITO substrate, Au NEAs, and graphene-Au NEAs, respectively. c) Cyclic voltammogram of P-aminophenyl phosphate (PAPP) on graphene-Au NEAs before and after enzyme reaction with alkaline phosphatase (ALP). d) Anodic peak (oxidation potential: IPA) value change achieved from of cyclic voltammogram of PAPP, before and after enzyme reaction (ALP), on bare ITO substrate and graphene-Au hybrid NEAs. e) The linear correlations between concentrations of ALP and the current signal at oxidation potential (IPA) of cyclic voltammetry. f) Schematic illustration of electrochemical signal change between undifferentiated and differentiated (osteocyte) hMSCs based on ALP generation. g) Cyclic voltammetry, and h) calculated Ipc values from time-dependent monitoring (range from D1 to D21) of hMSCs during osteogenic differentiation. 8.0 × 103 cells are seeded on 0.4 cm2 area and treated with osteogenic differentiation medium (OM). The medium was changed after each electrochemical measurement.
