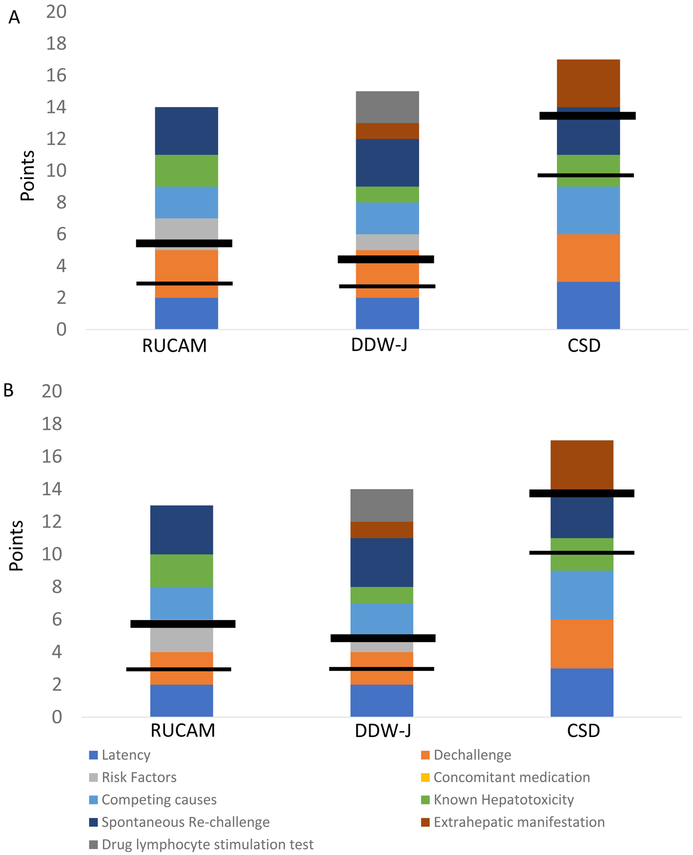
Figure 1. Comparison of point distributions for causality instruments.
The figure depicts the maximal points given in each category for RUCAM, DDW-J and CDS, respectively. The horizontal lines indicate the points required to qualify for possible (thinner line) and probable (thicker line) DILI, respectively. In (A) is shown a hepatocellular injury pattern and in (B), mixed/cholestatic injury pattern. Of note, the category for concomitant medications is unique to RUCAM and gives negative points depending on the strength of concern that an alternative drug may have causes the liver injury. This category is ignored should there be identification of a competing cause such as acute viral hepatitis. Additionally, note CDS maximum was adjusted to 17, as 20 points in unattainable, since it is not possible to be “on” a drug and “off” a drug at the same time.
Abbreviations: CDS: Clinical diagnostic scale as proposed by Maria & Victorino, DDW-J: a modified version of RUCAM developed during Japanese Digestive Disease Week, HC: hepatocellular, MC: mixed/cholestatic, RUCAM.