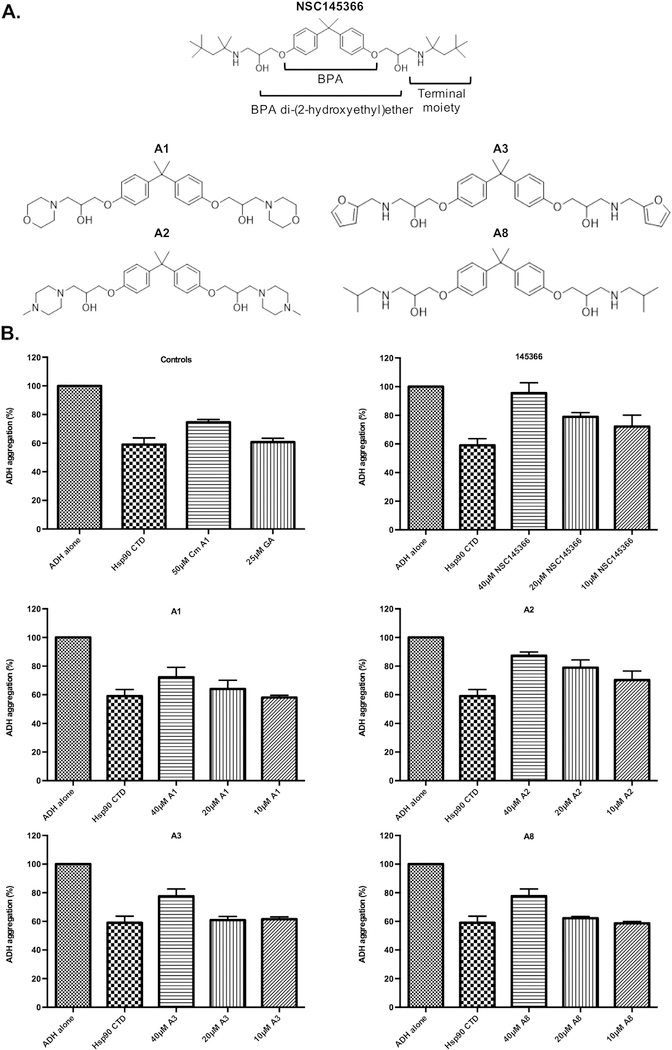
Fig. 7.
Inhibition of Hsp90 CTD chaperone activity is driven by BPA di-(2-hydroxyethyl)ether. A) Structure of NSC145366 and four symmetrical analogs (A1, A2, A3 and A8); all contain the same Bisphenol A (BPA) di-(2-hydroxyethyl) ether core but differ in their terminal moieties. B) Inhibitor activity of the analogs in the ADH chaperone assay. Cm A1 = coumermycin A1. All assays used identical conditions including 500 nM of Hsp90 CTD. *significant between untreated (Hsp90 C-terminus alone) and compound treated groups P ≤ 0.02 ‡significant between NSC145366 and analog groups P ≤ 0.003.