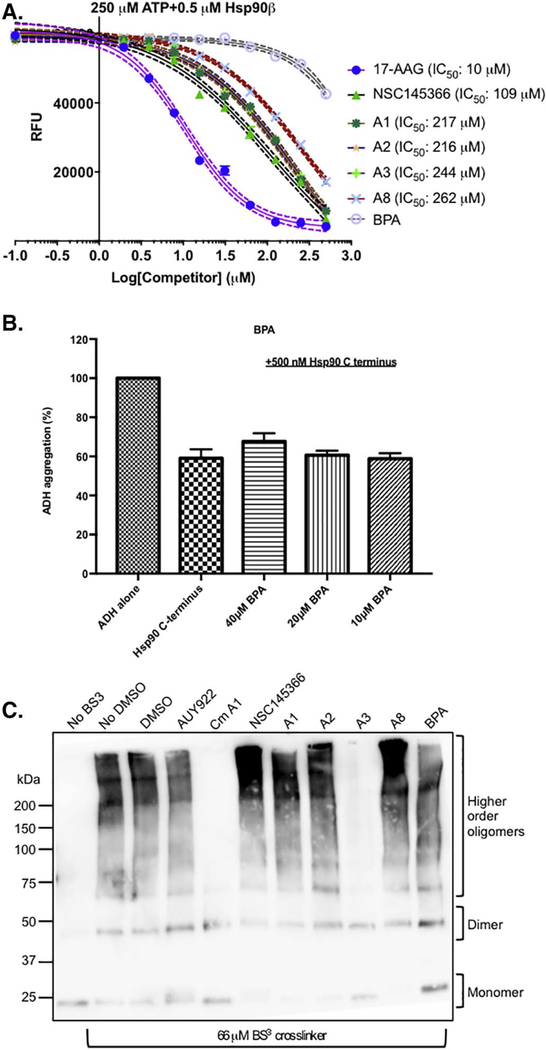
Fig. 8.
The BPA di-(2-hydroxyethyl) ether drives allosteric modulation of Hsp90 ATPase function in the context of side chains but BPA alone is not sufficient for inhibitory activity. A) Inhibition of ATPase activity by the analogs compared to NSC145366 and BPA. IC50 for each analog is shown. BPA does not have a measurable IC50. N = 3 with SEM error bars. B) ADH aggregation assay examining the ability of BPA to inhibit ADH aggregation. Minimal inhibition occurs at 40 μM. C) Crosslinking assay demonstrating the oligomerization of His6-Hsp90β CTD in the presence of all analogs and compounds. 66 μM of BS3 crosslinker was used in these samples. An identical experiment using 200 μM of BS3 (Fig. S10) enabled improved visualization of the A3-mediated dimer disruption.