Abstract
Introduction
In the scientific literature, there is very limited empirical information on end-of-life issues after stroke in the scientific literature. The present nationwide study describes the circumstances surrounding deaths that occur within a year after a stroke.
Patients and methods
Datasets from three nationwide Swedish registers (on stroke, palliative care and cause of death) were linked. Basic information was available for 42,502 unselected cases of death that occurred within a year after a stroke and more detailed information was available for 16,408 deaths. Odds ratios for characteristics of end-of-life care were calculated by logistic regression.
Results
In the late phase after stroke (three months to one year), 46% of patients died in a nursing home, whereas 37% of patients died in a hospital after readmission and 10% of patients died at home. Eleven per cent of deaths were reported as being unexpected. A next of kin was present at 49% of deaths. The frequency of unattended deaths (neither next of kin nor staff were present at the time of death) ranged from 5% at home with specialised home care to 25% in hospitals.
Discussion
This is, by far, the largest study published on end-of-life issues after stroke. Major differences between countries in healthcare, community services, family structure and culture may limit direct transfer of the present results to other settings.
Conclusion
There is considerable discordance between presumed ‘good death’ late after stroke (dying at home surrounded by family members) and the actual circumstances at the end of life.
Keywords: Stroke, end of life, terminal care, death, next of kin, nursing homes
Introduction
In most countries, advances in acute stroke care, rehabilitation and secondary prevention have resulted in reduced stroke mortality over recent decades.1 However, stroke remains one of the leading causes of death and is the underlying cause in approximately 1 in 10 deaths worldwide.1
Because stroke is one of the most common causes of death, it may seem paradoxical that so little empirical information related to end-of-life issues after stroke is available in the scientific literature. Respiratory distress and pain have been identified as the most disturbing symptoms at the end of life, and symptom control at the end of life has been reported as insufficient.2–5
It is a common perception that people prefer to die at home with their family members present.6–9 In the present study, we hypothesised that there is considerable discordance between this presumed ‘good death’ and the actual circumstances at the end of life late after stroke. We have related the place of death to key issues at the end of life during the first year after stroke with special emphasis on unaccompanied death, i.e. dying without any next of kin or member of the care team present at the time of death. This study used datasets from three nationwide Swedish registers. Basic information was available in 42,502 unselected cases of death after stroke, and more detailed information was available for more than 16,408 deaths.
Methods
Data sources and study population
Information from three major databases, the Swedish Stroke Register Riksstroke, the Swedish Register of Palliative Care (SRPC) and the national Cause of Death Register, were linked using unique personal identification numbers. Immediately after linkage, the combined database was de-identified by removing names, personal identification numbers and addresses.
Riksstroke is a national hospital performance register in which all hospitals admitting acute stroke patients in Sweden participate. Riksstroke covers an estimated 94% of all acute stroke patients treated in Swedish hospitals. A description of the register, information on coverage and details on what information is collected and its validity are available at the Riksstroke website (see http://www.riksstroke.org/eng/ and Riksstroke10).
The SRPC is a national quality register of the care of patients during the final week of life regardless of age, place of care and diagnosis (deaths that do not involve formalised palliative care are also covered).11 The registry includes data on place of death, symptoms, treatments and other circumstances during the final week of life. An English version of the items covered by the registry is available in Lundstrom et al.11 In 2016, the database covered 64% of all deaths in Sweden.12 The coverage varied between counties from 52% to 76% with no obvious geographical pattern.12 The coverage of stroke deaths is reported in the Results section.
The Swedish Cause of Death Register, managed by the National Board of Health and Welfare, has covered an estimated 99% of all deaths in the country.13 The Board adheres to the ICD-10 rules and guidelines for mortality and morbidity coding.14 A single underlying cause of death is recorded. For instance, if a physician records a complication to acute stroke, such as pneumonia, as the immediate cause of death, stroke is still recorded as the underlying cause of death, and this has been used in the present study. When acute stroke diagnoses recorded in the Cause of Death Register were validated, the positive predictive value was 78%.15
The primary analyses in this study include all stroke patients (ICD 10-codes: I61, I63, or I64) ≥18 years that were recorded in Riksstroke from 2007 to 2014 and who died within a year of the index stroke. The index stroke could be either a first-ever or a recurrent stroke event, but each individual was included only once. For description of place of death in relation to time after stroke, only patients recorded up to 2013 were included, permitting a minimum follow-up time of one year.
Data from the SRPC were used to describe details of end-of-life care. This included the care team’s assessment of whether the death was expected or unexpected, whether intravenous fluids and tube feeding were used at the time of death, whether the place of death complied with the last request stated by the person, date that the last examination by a physician before death was performed, and the presence of next of kin and/or staff at the time of death.
This study was approved by the Umeå Regional Ethics Review Board (No. 2015-247-32M).
Variable definitions
In Riksstroke, diagnoses of previous stroke and diabetes were based on medical records and atrial fibrillation from a previous diagnosis or atrial fibrillation from a new detection during hospital care. Hypertension was defined as being on antihypertensive treatment.
In the SRPC, next of kin included family members, a partner, relatives, close friends and neighbours.
Information on time from index stroke to death was obtained from Riksstroke (which updates its information on survival using the Population Register at The Swedish Tax Agency once a month) and information on underlying cause of death and place of death was retrieved from the Cause of Death Register.
Statistical methods
Differences in continuous variables were analysed using Student’s t-test, and categorical variables were analysed using the chi square test. Differences in end-of-life care and the presence of others at the time of death were analysed using logistic regression. Information on the presence of others at the time of death was missing in 3.1% of patients; no other variable exceeded 1% of missing data. Missing data were excluded from the analysis. Statistical analyses were performed in IBM SPSS Statistics version 24.
Results
Characteristics of patients
During the years 2007–2014, 201,969 patients were recorded in the Riksstroke register. During the same period, there were 42,502 deaths recorded within a year after the index stroke (one-year case fatality: 21%). Of these deaths, information on end-of-life care was available from the SRPC in 16,408 patients (39%).
The characteristics of the patients who died in the first year after an index stroke are shown in Table 1. There was a modest female majority, and the mean age at death was 82.3 years. The proportions of first-ever and recurrent stroke were 76% and 22%, respectively (2% missing information). There was considerable comorbidity with atrial fibrillation, diabetes and hypertension.
Table 1.
Characteristics of all included deceased stroke patients who died within one year after an index stroke and of the subpopulations with and without data on end-of-life care.
| Patient characteristics | All deceased (n = 42,502) | Data on end-of-life care (n = 16,408) | No data on end-of- life care (n = 26,094) | p |
|---|---|---|---|---|
| Sex | ||||
| Male | 18,803 (44%) | 7075 (43%) | 11,728 (45%) | <0.001 |
| Female | 23,699 (56%) | 9333 (57%) | 14,366 (55%) | |
| Mean age, years | ||||
| At death | 82.3 | 83.1 | 81.8 | <0.001 |
| Living alonea | ||||
| Yes | 26,637 (63%) | 10,593 (65%) | 16,044 (62%) | <0.001 |
| No | 15,427 (36%) | 5671 (35%) | 9756 (37%) | |
| Information missingc | 438 (1%) | 144 (1%) | 294 (1%) | |
| Comorbiditya | ||||
| Previous stroke | ||||
| Yes | 9351 (22%) | 3162 (19%) | 6189 (24%) | <0.001 |
| No | 32,411 (76%) | 13,021 (79%) | 19,390 (74%) | |
| Information missingc | 740 (2%) | 225 (1%) | 515 (2%) | |
| Atrial fibrillation | ||||
| Yes | 17,786 (42%) | 6925 (42%) | 10,861 (42%) | 0.588 |
| No | 24,073 (57%) | 9310 (57%) | 14,763 (57%) | |
| Information missingc | 643 (2%) | 173 (1%) | 470 (2%) | |
| Diabetes | ||||
| Yes | 9283 (22%) | 3597 (22%) | 5686 (22%) | 0.987 |
| No | 32,709 (77%) | 12,671 (77%) | 20,038 (77%) | |
| Information missingc | 510 (1%) | 140 (1%) | 370 (1%) | |
| Hypertension | ||||
| Yes | 26,122 (62%) | 10,544 (64%) | 15,578 (60%) | <0.001 |
| No | 15,486 (36%) | 5624 (34%) | 9862 (38%) | |
| Information missingc | 894 (2%) | 240 (2%) | 654 (3%) | |
| Level of consciousness on admission for index strokea | ||||
| Fully conscious | 23,250 (55%) | 9675 (59%) | 13,575 (52%) | <0.001 |
| Drowsy | 11,227 (26%) | 4169 (25%) | 7058 (27%) | |
| Unconscious | 7321 (17%) | 2259 (14%) | 5062 (19%) | |
| Information missingc | 704 (2%) | 305 (2%) | 399 (2%) | |
| Place of deathb | ||||
| In hospital | 26,504 (62%) | 9199 (56%) | 17,305 (66%) | <0.001 |
| In nursing home | 11,121 (26%) | 5543 (34%) | 5578 (21%) | |
| At home | 1617 (4%) | 629 (4%) | 988 (4%) | |
| Other, unknown | 3260 (8%) | 1037 (6%) | 2223 (9%) |
Note: P values indicate chi-square testing of differences between all deceased patients vs. patients with data available at the end of life, excluding patients with missing information.
Recorded at index stroke in the Riksstroke register.
As recorded in the Cause of Death register.
Excluded from test.
Table 1 also describes the characteristics of the subpopulations with and without detailed information from the SRPC on end-of-life care. Patients recorded in the SRPC had a similar age distribution and a similar comorbidity pattern as the entire group of deceased patients. Because of the large number of patients, several differences in clinical parameters were statistically significant. The proportion of patients who died in the hospital was lower, and the proportion who died in a nursing home was higher among patients recorded in the SRPC than in the total cohort.
The underlying causes of death according to the Cause of Death Register are shown in Supplementary Table 1. Over the first year, 54% of patients reported cerebrovascular disease as the underlying cause of death and 29% reported another vascular disease as the underlying cause of death.
Where do stroke patients die?
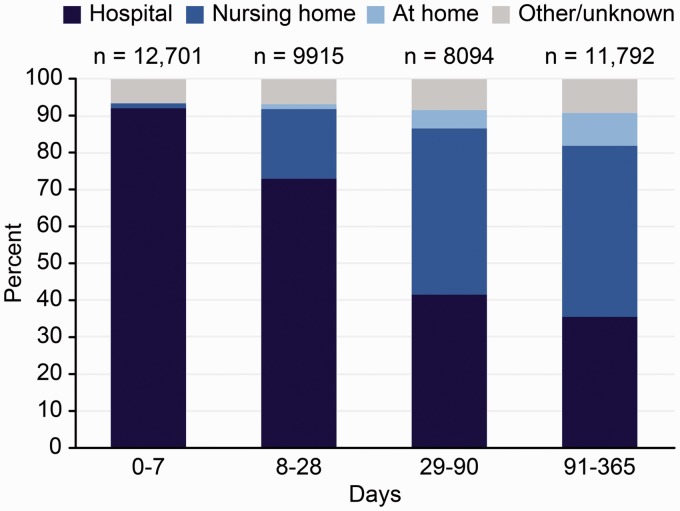
During the first year after an index stroke, the majority of patients died in the hospital (62%). As shown in Figure 1, although the proportion of patients who died in the hospital declined during the first year, this proportion was as high as 36% in the three-month to one-year interval after an index stroke. After an index stroke, only 63 of 42,502 patients (0.1%) stayed in the hospital for more than 90 days, including geriatric wards, indicating that nearly every in-hospital death in the three-month to one-year interval occurred after re-admission. At more than three months after a stroke, 46% of patients died in a nursing home. The proportion who died in their own homes increased along with increasing time since first-ever stroke, but even in patients who died late after stroke (three months to one year), the proportion was low (9%). Very few patients died in palliative in-patient care.
Figure 1.
Place of death at different times after index stroke. Patients recorded in Riksstroke during 2007–2013 and followed until to the end of 2014 (n = 42,502).
The mean age of patients who died in nursing homes (85.6 years) was 4.4 years higher on average than that of patients who died in the hospital (81.2 years) and was and was 6.4 years higher than that of patients who died in their own homes (79.2 years).
Patient characteristics and end-of-life issues
Table 2 shows selected characteristics of end-of-life care in relation to place of death, according to information in the SRPC. One caveat is that there were a high proportion of patients with no information available for some of the variables; for these patients, the proportions shown should be regarded as minimum estimates.
Table 2.
Characteristics of end-of-life care in deceased patients during the years 2007 through 2014 who had died within a year from the index stroke and had data both in the Riksstroke register and in the Swedish Register of Palliative Care (n = 16,408).
| Own home | In hospital | Nursing home | Other, unknown | All | |
|---|---|---|---|---|---|
| Expected death | |||||
| Yes | 545 (87%) | 7791 (85%) | 4798 (87%) | 852 (82%) | 13,986 (85%) |
| No | 69 (11%) | 967 (10%) | 567 (10%) | 129 (12%) | 1732 (11%) |
| No information | 15 (2%) | 441 (5%) | 178 (3%) | 56 (5%) | 690 (4%) |
| Odds ratio (95% CI) | Ref. | 0.77 (0.60–0.98) | 0.88 (0.69–1.13) | 0.64 (0.48–0.85) | – |
| Response to the question ‘Did the place of death comply with the request last stated by the person?’ | |||||
| Yes | 489 (78%) | 554 (6%) | 2132 (39%) | 251 (24%) | 3426 (21%) |
| No or no information | 140 (22%) | 8645 (94%) | 3411 (61%) | 786 (76%) | 12,982 (79%) |
| Odds ratio (95% CI) | Ref. | 0.02 (0.02–0.03) | 0.19 (0.16–0.23) | 0.10 (0.08–0.13) | – |
| Intravenous fluid or tube feeding during last day of life | |||||
| Yes | 11 (2%) | 2415 (26%) | 299 (5%) | 177 (17%) | 2902 (18%) |
| No | 414 (66%) | 4163 (45%) | 3414 (62%) | 543 (52%) | 8534 (52%) |
| No information | 204 (32%) | 2621 (29%) | 1830 (33%) | 317 (31%) | 4972 (30%) |
| Odds ratio (95% CI) | Ref. | 21.9 (11.7–41.1) | 3.9 (2.0–7.3) | 13.1 (6.9–25.0) | – |
| Last examination before death by a physician | |||||
| Day(s) | 219 (35%) | 6550 (71%) | 2149 (39%) | 557 (54%) | 9475 (58%) |
| Week(s) | 156 (25%) | 49 (1%) | 1224 (22%) | 131 (13%) | 1560 (10%) |
| A month or more | 43 (7%) | 7 (0%) | 259 (5%) | 22 (2%) | 331 (2%) |
| No information | 211 (33%) | 2593 (28%) | 1911 (34%) | 327 (31%) | 5042 (30%) |
| Odds ratio (95% CI) | Ref. | 4.33 (3.64–5.15) | 1.13 (0.94–1.34) | 2.07 (1.68–2.54) | – |
Note: Logistic regression analysis with dependent variables coded yes/no or no information and as day(s)/all other response alternatives. Adjusted for gender, age and level of consciousness on admission to hospital (as a proxy for stroke severity).
Of the patient deaths, 10–12% were found to be unexpected, regardless of whether death occurred in a hospital, a nursing home or at home. The proportion of patients who died in the hospital for whom ‘yes’ was reported on the question ‘Did the place of death comply with the request last stated by the person?’ was only 6%, mainly because the patient’s request was not known. This proportion was much higher in patients who died in nursing homes (39%) and was even higher in patients who died at home (78%). The 95% confidence intervals of odds ratios for the different places of death were non-overlapping.
Although intravenous fluid supply or tube feeding was relatively common during the final days of life in patients who died in the hospital, these procedures were uncommon in patients who died in nursing homes and were even more uncommon in patients who died at home (Table 2).
Of patients who died in the hospital, 1% was not examined by a physician in the week preceding their death. The proportions for patients who died in nursing homes and at home were 22 and 25%, respectively (Table 2).
Unattended death
Overall, a next of kin was present at the time of death in half of the cases; however, the presence of a next of kin declined markedly with increasing patient age, but the presence of a staff member increased with patient age (Table 3). The proportion of patients who died without any other person present (unattended death) was 20%, which was very similar in men and women (Table 3). Unattended death increased with increasing age from 11% for patients aged below 55 years to 22% for patients aged above 85 years. After multiple adjustments in the regression model, the differences were statistically significant between patients who died at <55 years old vs. all age groups ≥65 years old.
Table 3.
Presence of another person at the time of death, n = 16,408.
| None n (%) | Next of kin (with or without staff present) n (%) | Staff only n (%) | Information missinga n (%) | Multiple logistic regression odds ratios (95% CI) | |
|---|---|---|---|---|---|
| All | 3315 (20) | 8106 (49) | 4481 (27) | 506 (3) | |
| Sex | |||||
| Men | 1452 (21) | 3506 (50) | 1914 (27) | 203 (3) | Ref. |
| Women | 1863 (20) | 4600 (49) | 2567 (28) | 303 (3) | 1.03 (0.95–1.11) |
| Age at death | |||||
| <55 | 19 (11) | 114 (66) | 37 (22) | 2 (1) | Ref. |
| 55–64 | 85 (16) | 288 (55) | 128 (25) | 19 (4) | 0.51 (0.34–1.02) |
| 65–74 | 360 (19) | 1057 (55) | 457 (24) | 59 (3) | 0.52 (0.32–0.87) |
| 75–84 | 983 (19) | 2652 (51) | 1403 (27) | 153 (3) | 0.53 (0.33–0.88) |
| ≥85 | 1868 (22) | 3995 (47) | 2456 (29) | 273 (3) | 0.48 (0.29–0.78) |
| Living conditionb | |||||
| Married/cohabitating | 954 (17) | 3341 (59) | 1232 (22) | 144 (3) | Ref. |
| Single | 2337 (22) | 4696 (44) | 3200 (30) | 359 (3) | 0.73 (0.67–0.80) |
| Information missinga | 24 (17) | 69 (48) | 49 (34) | 2 (1) |
Note: Multiple regression logistic analysis includes all variables listed in the table.
Excluded from test.
Recorded at the time of index stroke.
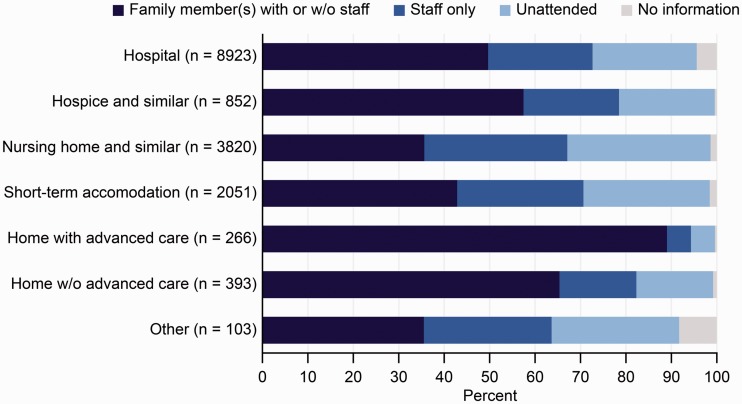
As shown in Figure 2, the proportion of patients with an unattended death was highest among those who died in the hospital (25%) and was lowest in patients who died with specialised home care (5%). The proportion of patients with a next of kin present was highest in patients who died in specialised home care (90%) and lowest in patients who died in a nursing home (45%) (Figure 2). This proportion was considerably higher in patients who were married or cohabitating (59%) than in patients who were single (44%, Table 3). The differences were statistically significant in the multiple regression model. Additionally, 30% of single patients had a staff member present when they did not have a next of kin present, compared to 22% of non-single patients. Overall, the proportion of unattended death was higher in single (22%) patients than in married/cohabitating patients (17%).
Figure 2.
Presence of next of kin and staff at the time of death and unattended death by type of care. Based on data from 16,408 patients in the Swedish Register of Palliative Care (SRPC). Information on presence of another person was missing in 3315 decedents.
Sensitivity analysis
In the principle analyses, patients with first-ever and recurrent stroke were analysed together. When key variables in the population of patients with first-ever strokes were analysed separately, there were only marginal differences from those of the entire patient population (Supplementary Table 2).
Discussion
The present results show that, in Sweden, the majority of patients who die between one month and one year after a stroke are living in nursing homes and that only 1 in 10 deaths occur at home during this period. In the 3–12-month interval after stroke, more than a third of deaths occur in the hospital, usually after readmission. One in 10 patients who died in nursing homes or at home were not examined by a physician in the month preceding death. The frequency of unattended deaths ranged from 5% at home with specialised home care to 25% in hospitals.
As described in the Introduction section, our study is not the first to assess end-of-life issues after stroke; however, it is by far the largest one. By combining information from several large databases, it was possible to obtain a comprehensive nationwide representation of the subject.
It is commonly presumed that for most severely ill patients, the preferred setting for death is at home in the presence of loved ones with whom they have strong relationships.6–8 Studies on patients, family members and healthcare providers have also shown that control, maintenance of independence and respect for the patient’s preferences regarding the death process are considered important components of a ‘good death’.7,16
A recent systematic review of the qualitative evidence showed that most family caregivers preferred home care.9 If this preference is considered ideal, there is a strong discordance between patient preference and the actual place of death after stroke. The proportion of stroke patients who died at home (4%) is lower than the corresponding proportion of all patients in the SRPC (11% in 2011–201412). Even late after stroke, only 1 in 10 deaths occurred at home in Sweden. There is also a very low concordance between the preferred and actual sites of death in the US6 and the UK.17 The proportion of stroke patients readmitted to acute care in a hospital at the end of life is conspicuously high (36% in the three-month to one-year interval after an index stroke). This may indicate inferior access to high-quality palliative care that ensures confidence in the care provided at home or in nursing homes. Unrealistic expectations of what can be achieved by acute care interventions in frail patients after stroke may also contribute.
As discussed by Fischer et al.,6 it seems that the more predictable the trajectory (such as in terminal cancer), the higher the concordance between preferred and actual site of death. The trajectories of frail stroke patients often involve stroke recurrences, other acute cardiovascular events or other severe comorbidities, i.e. events that are difficult to predict. This may partly explain why more than a third of stroke patients die in acute care hospitals as late as 3–12 months after the stroke. It is also likely that family resources and community home care support prove to be insufficient in making end-of-life home care a realistic option. It may be that when faced with a highly pressing situation at the end of life, death in an institution may be accepted or even preferred. It should also be noted that in a study based on the SRPC, transition to end-of-life care had been discussed in only 15% of the stroke patients who eventually died, although self-determination was retained until the last days in 73% of patients.3
As we have previously observed in cancer patients,18 there is a decline in the presence of next of kin at the time of death with the increasing age of the patient. This decline is partly because fewer decedents have spouses that are still alive. By far, the highest proportion of next of kin present was in patients with specialised home care. Efforts to strengthen family involvement is an integral part of specialised home care, as with other forms of palliative care.19
Our study has some limitations. In all registers that we used, data were collected during routine clinical practice. Although the coverage is nearly complete in the Cause of Death Register (99%13) and in Riksstroke (94%10), the present data indicate a 39% coverage of deaths within a year after stroke in the SRPC. Age and sex distribution and comorbidity patterns in patients recorded in the SRPC differed only slightly compared with the total cohort of deceased patients. Therefore, it seems likely that the more detailed information in our analyses is reasonably representative of all patients who died within the first year after stroke in Sweden. Patients who died in nursing homes were overrepresented in the SRPC; this would not influence the description of end of life in each of the different settings.
The inclusion of cases in the present study and much of the patient background information was based on the Riksstroke register. This database has been extensively validated, with data quality sufficient for clinical epidemiology studies (for an overview, see Riksstroke10). A validation study of stroke diagnoses in the Swedish Cause of Death register showed a 22% or higher frequency of false-positive diagnoses.15 Other causes of death have only partly been validated. Our data on cause of death (Supplementary Table 1) should therefore be regarded with caution. The information in the SRPC has been validated.20 None of the questions with a low congruity between the register and the medical record information were used in the present analyses.
Our results may be valid for the Swedish setting, but major differences between countries in healthcare, community services, family structure and culture make it problematic to directly transfer our results to other countries. In countries with more traditional family roles, institutional care of patients with severely disabling stroke is less common,21 and it is likely that a higher proportion of deaths occur at home with next of kin present when death occurs.
We conclude that there is considerable discordance between presumed ‘good death’ late after stroke (dying at home surrounded by next of kin) and the actual circumstances at the end of life. Only a small minority of patients die at home. It may be that when faced with a highly pressing situation at the end of life, death in an institution is accepted or even preferred. Many patients die without a next of kin present; in fact, many deaths during the first year after stroke are unattended.
Supplementary Material
Acknowledgements
We would like to thank Professor Andreas Terént and Dr Greger Fransson who participated in the early planning of this study. Maria Hals Berglund provided statistical assistance. Marie Eriksson gave advice and support throughout the study. The Riksstroke and SRPC Steering Groups generously provided access to the registers.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The SRPC and Riksstroke are national quality registers funded by the Swedish Association of Local Authorities and Regions and the Ministry of Health.
Ethical approval
Ethical approval for this study was obtained from the Umeå Regional Ethics Review Board (REC number: 2015-247-32M).
Informed consent
Informed consent was not sought for the present study because. All patients were dead and all data was completely untraceable on an individual level after the merging. The whole merging procedure was performed at the National Board of Health and Welfare (Socialstyrelsen), Stockholm, Sweden. So for all data used all information is anonymised.
Guarantor
BS.
Contributorship
KA and BS researched literature, gaining ethical approval, development, and data analysis. SL was involved the discussion and provided access to some of the data needed. KA wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
References
- 1.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 2014; 383: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Addington-Hall J, Lay M, Altmann D, et al. Symptom control, communication with health professionals, and hospital care of stroke patients in the last year of life as reported by surviving family, friends, and officials. Stroke 1995; 26: 2242–2248. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson H, Milberg A, Hjelm K, et al. End of life care for patients dying of stroke: a comparative registry study of stroke and cancer. PLoS One 2016; 11: e0147694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazzocato C, Michel-Nemitz J, Anwar D, et al. The last days of dying stroke patients referred to a palliative care consult team in an acute hospital. Eur J Neurol 2010; 17: 73–77. [DOI] [PubMed] [Google Scholar]
- 5.Ntlholang O, Walsh S, Bradley D, et al. Identifying palliative care issues in inpatients dying following stroke. Ir J Med Sci 2016; 185: 741–744. [DOI] [PubMed] [Google Scholar]
- 6.Fischer S, Min SJ, Cervantes L, et al. Where do you want to spend your last days of life? Low concordance between preferred and actual site of death among hospitalized adults. J Hosp Med 2013; 8: 178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meier EA, Gallegos JV, Thomas LP, et al. Defining a good death (successful dying): Literature review and a call for research and public dialogue. Am J Geriatr Psychiatry 2016; 24: 261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singer PA, Martin DK, Kelner M. Quality end-of-life care: patients' perspectives. JAMA 1999; 281: 163–168. [DOI] [PubMed] [Google Scholar]
- 9.Woodman C, Baillie J, Sivell S. The preferences and perspectives of family caregivers towards place of care for their relatives at the end-of-life. A systematic review and thematic synthesis of the qualitative evidence. BMJ Support Palliat Care 2016; 6: 418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riksstroke. Evaluations of variables in Riksstroke, the Swedish Stroke Register. Short version in English, www.riksstroke.org/wp-content/uploads/2015/06/Evaluations-of-variables-in-Riksstroke-rev-15-08-03.pdf (2015, accessed 5 June 2017).
- 11.Lundstrom S, Axelsson B, Heedman PA, et al. Developing a national quality register in end-of-life care: the Swedish experience. Palliat Med 2012; 26: 313–321. [DOI] [PubMed] [Google Scholar]
- 12.Svenska Palliativregistret. Årsrapport (Annual reports), http://media.palliativ.se/2017/03/Svenska-palliativregistret-%C3%85rsrapport-2017.pdf (accessed 5 June 2017).
- 13.Socialstyrelsen. Dödsorsaker 2014 (Causes of Death 2014, summary in English). Offical Statistics of Sweden, www.socialstyrelsen.se/publikationer2016/2016-8-3 (2014, accessed 5 June 2017).
- 14.World Health Organization. International Statistical Classification of Diseases and Related Health Problems (ICD-10), 10th Revision, Volume 2 Instruction manual, sections 4.1 and 4.2, www.who.int/classifications/icd/ICD10Volume2_en_2010.pdf?ua=1 (2010, accessed 12 September 2017).
- 15.Koster M, Asplund K, Johansson A, et al. Refinement of Swedish administrative registers to monitor stroke events on the national level. Neuroepidemiology 2013; 40: 240–246. [DOI] [PubMed] [Google Scholar]
- 16.Rietjens JA, van der Heide A, Onwuteaka-Philipsen BD, et al. Preferences of the Dutch general public for a good death and associations with attitudes towards end-of-life decision-making. Palliat Med 2006; 20: 685–692. [DOI] [PubMed] [Google Scholar]
- 17.Hunt KJ, Shlomo N, Addington-Hall J. End-of-life care and preferences for place of death among the oldest old: results of a population-based survey using VOICES-Short Form. J Palliat Med 2014; 17: 176–182. [DOI] [PubMed] [Google Scholar]
- 18.Lindskog M, Tavelin B, Lundstrom S. Old age as risk indicator for poor end-of-life care quality – a population-based study of cancer deaths from the Swedish Register of Palliative Care. Eur J Cancer 2015; 51: 1331–1339. [DOI] [PubMed] [Google Scholar]
- 19.National Consensus Project for Quality Palliative Care. Clinical practice guidelines for quality palliative care, 3rd ed. www.hpna.org/multimedia/NCP_Clinical_Practice_Guidelines_3rd_Edition.pdf (2013, accessed 12 September 2017).
- 20.Martinsson L, Heedman PA, Lundstrom S, et al. Validation study of an end-of-life questionnaire from the Swedish Register of Palliative Care. Acta Oncol 2011; 50: 642–647. [DOI] [PubMed] [Google Scholar]
- 21.Asplund K, Ashburner S, Cargill K, et al. Health care resource use and stroke outcome. Multinational comparisons within the GAIN International trial. Int J Technol Assess Health Care 2003; 19: 267–277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.