Abstract
Purpose
Beyond intravenous thrombolysis, evidence is lacking on acute treatment of minor stroke caused by large artery occlusion. To identify candidates for additional endovascular therapy, we aimed to determine the frequency of non-haemorrhagic early neurological deterioration in patients with intravenous thrombolysis-treated minor stroke caused by occlusion of large proximal and distal cerebral arteries. Secondary aims were to establish risk factors for non-haemorrhagic early neurological deterioration and report three-month outcomes in patients with and without non-haemorrhagic early neurological deterioration.
Method
We analysed data from the SITS International Stroke Thrombolysis Register on 2553 patients with intravenous thrombolysis-treated minor stroke (NIH Stroke Scale scores 0–5) and available arterial occlusion data. Non-haemorrhagic early neurological deterioration was defined as an increase in NIH Stroke Scale score ≥4 at 24 h, without parenchymal hematoma on follow-up imaging within 22–36 h.
Findings
The highest frequency of non-haemorrhagic early neurological deterioration was seen in 30% of patients with terminal internal carotid artery or tandem occlusions (internal carotid artery + middle cerebral artery) (adjusted odds ratio: 10.3 (95% CI 4.3–24.9), p < 0.001) and 17% in extracranial carotid occlusions (adjusted odds ratio 4.3 (2.5–7.7), p < 0.001) versus 3.1% in those with no occlusion. Proximal middle cerebral artery-M1 occlusions had non-haemorrhagic early neurological deterioration in 9% (adjusted odds ratio 2.1 (0.97–4.4), p = 0.06). Among patients with any occlusion and non-haemorrhagic early neurological deterioration, 77% were dead or dependent at three months.
Conclusions
Patients with minor stroke caused by internal carotid artery occlusion, with or without tandem middle cerebral artery involvement, are at high risk of disabling deterioration, despite intravenous thrombolysis treatment. Acute vessel imaging contributes usefully even in minor stroke to identify and consider endovascular treatment, or intensive monitoring at a comprehensive stroke centre, for patients at high risk of neurological deterioration.
Keywords: Cerebral infarct, thrombolysis, stroke severity, stroke management
Introduction
Patients with acute minor stroke caused by large artery occlusion pose a particular challenge in acute clinical decision making. Intravenous thrombolysis (IVT) is the mainstay therapy for acute ischaemic stroke regardless of severity, with firm evidence of benefit in cases with relatively mild symptoms.1 Large cerebral artery occlusions typically lead to more severe stroke.2,3 However, in the presence of a complete circle of Willis and a well-developed collateral circulation, patients with proximal arterial occlusions may at times present with only relatively mild symptoms. It is a well known clinical phenomenon that such patients can suffer significant clinical deterioration as the initial hours pass.4–8 A common mechanism behind this course of events is infarct growth beyond the initial infarct zone, due to collateral circulation failure, thrombus extension, or secondary thromboembolism.9–13 Early neurological deterioration (END), predominantly defined in literature as an increase in NIH Stroke Scale (NIHSS) score by 4 or more points, is strongly related to poor long-term functional outcome.14,15 Radiologically proven arterial occlusion in patients with minor stroke is a well-established predictor of early deterioration and disability.16,17 However, it is still unclear which patients with minor stroke, and which occluded arterial segments, have the highest risk of deterioration despite IV tPA and could potentially be spared disability through early endovascular therapy. The randomised controlled trials of mechanical thrombectomy published in 2015 excluded, or enrolled very few, patients with NIHSS score <6, thus unable to address efficacy and safety of treatment in this group.18 The 2015 American Stroke Association guideline update expressed that endovascular therapy ‘may be reasonable’ in minor stroke caused by internal carotid artery (ICA) or proximal part of the middle cerebral artery (MCA-M1) occlusion, making no mention of patients with minor stroke caused by other arterial occlusions.19 Considering a potential reluctance among clinicians to recommend patients with minor stroke symptoms for an invasive procedure, the overarching aim of our present study is to facilitate the identification of patients in whom the initial ‘minor stroke’ presentation masks a more ominous condition.
The primary objective of this study was to determine the frequency of non-haemorrhagic early neurological deterioration (nhEND) in patients who receive IVT for minor stroke caused by occlusion of specific cerebral arteries, including both large, proximal vessels and more distal ones. The secondary objectives were to establish risk factors for END and report three-month functional outcomes and mortality in patients with and without END.
Patients and methods
We analysed patient data recorded in the SITS International Stroke Thrombolysis Register (SITS-ISTR) between 1 December 2012 and 25 April 2016. Patients treated with IV alteplase (Actilyse, Boehringer-Ingelheim, Germany), with confirmed baseline data entered using the standard IVT case report form (N = 34,566) were considered. The study period was based on the time of implementation in the SITS database of optional variables for registering detailed information on sites of arterial occlusion using CT or MR angiography (CTA/MRA) performed at baseline. We further required availability of complete NIHSS evaluation at baseline and only included patients with baseline total NIHSS scores between 0 and 5 (n = 6860). Vessel occlusion was defined as complete occlusion or <50% filling of the vascular territory. Furthermore, patients who had any endovascular procedure (thrombectomy, extra- or intracranial stenting or angioplasty, intraarterial thrombolysis) performed during the acute hospital stay were excluded from the analysis in order to isolate the study population to patients who received only IVT as the causal therapy for arterial recanalisation. This exclusion was also necessitated by the fact that the SITS-ISTR IVT data form does not contain exact time points of performed endovascular treatments, making it impossible to discern if the procedures were done before or after the times of NIHSS evaluation (collected at baseline, at 2 h, and at 24 h after start of IVT).
The SITS-ISTR is an ongoing, prospective, academic-driven, multinational register for centres using thrombolysis for the treatment of acute ischaemic stroke. The methodology of the SITS-ISTR, including procedures for data collection and management, patient identification, and verification of source data, has been described previously.20,21 Consecutive case reporting is a basic commitment for participating in the SITS-ISTR. We collected baseline and demographic characteristics, stroke severity per the standard 11-item, 42-point NIHSS, time logistics, medication history, and imaging data on admission and follow-up. Assessment of imaging studies and neurological and functional status were done according to clinical routine at centres participating in the SITS-ISTR, with no additional central adjudication.
Ethics approval and data monitoring
Ethics approval was obtained from the Stockholm Regional Ethics Committee for this project as part of the SITS-MOST II study framework. Ethics approval and patient consent for participation in the SITS-ISTR were obtained in countries that required this; other countries approved the register for anonymised audit. The SITS International Coordination Office monitored the SITS-ISTR data online and checked individual patient data monthly to identify errors or inconsistencies.
Outcome measurements
nhEND was defined as increase in NIHSS score ≥4 within 24 h after IVT, with no parenchymal hematoma (PH) of any size or location on routine follow-up imaging at 22–36 h, or earlier, if clinically indicated. Functional outcomes and mortality were assessed at three-month follow-up using the modified Rankin Scale (mRS), with poor outcome defined as mRS 3–6.
Statistical analysis
We performed descriptive univariate statistics for baseline clinical and imaging data, as well as outcomes, comparing patients with and without nhEND. For continuous and ordinal variables, median and interquartile range values were calculated. For categorical variables, we calculated percentage proportions by dividing the number of events by the total number of patients, excluding missing or unknown cases, as done in previous SITS publications.21–23 For calculation of significance of difference between medians and proportions we used the Mann–Whitney U-test and the Pearson χ2 method, respectively. Furthermore, we calculated the sensitivity, specificity, positive and negative predictive values (PPV and NPV) of CTA/MRA at baseline to detect patients who would go on to develop nhEND. To investigate the association between nhEND and clinical and radiological parameters, multivariate logistic regression analysis was performed adjusting for baseline variables showing a positive association with nhEND at the p ≤ 0.10 level in univariate analysis. In this analysis, variables significant at p < 0.05 were regarded as independent risk factors for nhEND. Analyses were performed using STATISTICA 13.0 (Dell Inc., Tulsa, OK, USA).
Results
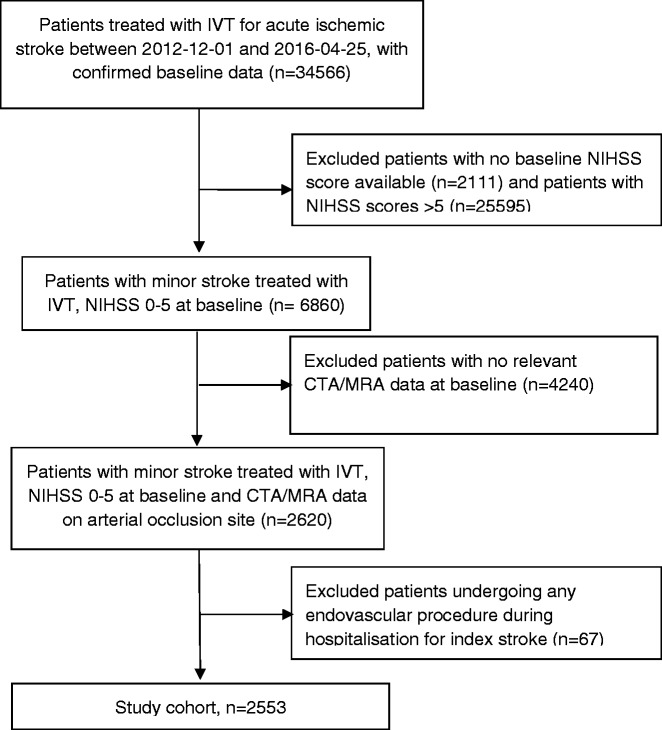
A total of 2553 patients from 241 hospitals in 34 countries comprised the study cohort (Figure 1). CTA was the baseline vessel imaging modality in 2437 (95.5%) cases and MRA in 116 (4.5%). The distribution of arterial occlusion sites among study patients is shown in Table 1.
Figure 1.
Patient selection flow diagram. CTA/MRA: CT or MR angiography; IVT: intravenous thrombolysis; NIHSS: NIH Stroke Scale
Table 1.
Arterial occlusion sites in patients with minor stroke treated with IVT.
| Site of occlusion | N = 2553 | Per cent of N | Per cent of all occlusions |
|---|---|---|---|
| ICA-T or ICA+MCA | 20 | 0.8 | 3.1 |
| ICA | 101 | 4.0 | 15.9 |
| ACA | 13 | 0.5 | 2.0 |
| MCA-M1 | 92 | 3.6 | 14.5 |
| MCA-M2 | 147 | 5.8 | 23.1 |
| MCA-M3 | 39 | 1.5 | 6.1 |
| PCA | 51 | 2.0 | 8.0 |
| Basilar | 32 | 1.3 | 5.0 |
| Vertebral | 62 | 2.4 | 9.8 |
| Site unspecified | 78 | 3.1 | 12.3 |
| Any occlusion | 635 | 24.9 | |
| No occlusion | 1918 | 75.1 |
ACA: anterior cerebral artery; ICA-T: terminal internal carotid artery; ICA+MCA: tandem ICA and middle cerebral artery occlusion; IVT: intravenous thrombolysis; M1, M2, M3: first, second, and third segments of the MCA; PCA: posterior cerebral artery.
Data from follow-up imaging at 22–36 h or earlier (if performed due to clinical indication) were available in 606/635 (95.4%) patients with arterial occlusion at baseline and 1810/1918 (94.4%) patients with no occlusion. Clinical follow-up data at 24 h were available in 2481/2553 patients (97.2%). PH (any size and location) was seen in 18/606 (3.0%) patients with occlusions and 55/1810 (3.0%) patients without occlusions, p = 0.93.
In patients without PH and with available 24 h neurological follow-up (n = 2336), END occurred in 53/587 (9.0%) of those with any arterial occlusion compared to 55/1749 (3.1%) of those with no occlusion, p < 0.001. Based on these numbers, we could calculate a sensitivity of 49.1%, specificity of 76.0%, PPV of 9.0%, and NPV of 96.9% for baseline vessel imaging to predict nhEND in patients treated with IVT for minor acute ischaemic stroke.
The highest frequency of nhEND at 30.0% was seen in patients who had terminal carotid (ICA-T) or tandem ICA + MCA occlusions, and the lowest in patients with M3-MCA and posterior cerebral artery (PCA) occlusions at 0 and 2.2%, respectively (Table 2).
Table 2.
Proportion of patients with non-haemorrhage-related END (nhEND) and adjusted odds ratios (aORs) for nhEND by site of arterial occlusion at baseline.
| Site of occlusion | nhEND n/N | nhEND % (95% CI) | P | aOR (95% CI) for nhEND | P |
|---|---|---|---|---|---|
| ICA-T or ICA+MCA | 6/20 | 30.0 (14.6–51.9) | <0.001 | 10.3 (4.3–24.9) | <0.001 |
| ICA | 16/96 | 16.7 (10.5–25.4) | <0.001 | 4.3 (2.5–7.7) | <0.001 |
| ACA | 2/12 | 16.7 (4.7–44.8) | 0.008 | 5.9 (1.6–22.1) | 0.008 |
| MCA-M1 | 8/86 | 9.3 (4.8–17.3) | 0.002 | 2.1 (0.97–4.4) | 0.061 |
| MCA-M2 | 8/137 | 5.8 (3.0–11.1) | 0.091 | 1.6 (0.8–3.1) | 0.14 |
| MCA-M3 | 0/35 | 0.0 (0.0–9.9) | 0.287 | 0.6 (0.1–4.2) | 0.57 |
| PCA | 1/46 | 2.2 (0.4–11.3) | 0.709 | 0.4 (0.1–3.2) | 0.41 |
| Basilar | 3/29 | 10.3 (3.6–26.4) | 0.030 | 2.0 (0.6–6.6) | 0.26 |
| Vertebral | 4/56 | 7.1 (2.8–17.0) | 0.098 | 2.3 (0.96–5.5) | 0.063 |
| Site unspecified | 5/70 | 7.1 (3.1–15.7) | 0.094 | 2.7 (1.3–5.7) | 0.007 |
| Any occlusion | 53/587 | 9.0 (7.0–11.6) | <0.001 | 2.5 (1.7–3.4) | <0.001 |
| No occlusion | 55/1749 | 3.1 (2.4–4.1) | Ref. | Ref. | Ref. |
ACA: anterior cerebral artery; ICA-T: terminal internal carotid artery; ICA+MCA: tandem ICA and middle cerebral artery occlusion; M1, M2, M3: first, second, and third segments of the MCA; PCA: posterior cerebral artery; nhEND: increase in NIHSS score ≥4 at 24 h with no PH on follow-up imaging within 22–36 h; Ref: reference. Patients with no visible arterial occlusion on baseline CTA/MRA were used as reference for comparison.
In the population with minor stroke and visible arterial occlusion, patients with and without nhEND had comparable medical history and baseline clinical parameters, with the exception of congestive heart failure, which was seen in 11% versus 5% (p = 0.049) (Table 3). Large artery atherosclerosis was somewhat more often the cause of stroke in patients with nhEND, at 56% versus 42.5%, trending towards significance (p = 0.066).
Table 3.
Clinical characteristics of patients with baseline arterial occlusion, with and without non-haemorrhage-related END (nhEND).
| nhEND, n = 53 |
No nhEND, n = 534 |
||||
|---|---|---|---|---|---|
| No./Total | Median (IQR) or % | No./Total | Median (IQR) or % | p Value | |
| Patient history | |||||
| Age | 53 | 67 (59–75) | 533 | 67 (57–76) | 0.97 |
| Sex (F) | 18/53 | 34.0% | 189/534 | 35.4% | 0.84 |
| AF | 4/53 | 7.5% | 88/532 | 16.5% | 0.11 |
| Heart failure | 6/53 | 11.3% | 26/532 | 4.9% | 0.049 |
| HT | 39/53 | 73.6% | 341/533 | 64.0% | 0.16 |
| DM | 14/53 | 26.4% | 91/532 | 17.1% | 0.09 |
| Hyperlipidaemia | 16/53 | 30.2% | 185/528 | 35.0% | 0.48 |
| Previous stroke | 4/53 | 7.5% | 46/531 | 8.7% | 0.78 |
| AP or OAC treatment | 18/53 | 34.0% | 208/534 | 39.0% | 0.48 |
| Current smoker | 13/53 | 24.5% | 85/509 | 16.7% | 0.15 |
| Previous smoker | 2/49 | 4.1% | 69/495 | 13.9% | 0.051 |
| Clinical parameters | |||||
| NIHSS | 53 | 4 (3–5) | 534 | 4 (3–5) | 0.12 |
| OTT | 52 | 165 (121–218) | 514 | 160 (125–210) | 0.57 |
| SBP | 51 | 160 (141–180) | 529 | 156 (140–170) | 0.11 |
| DBP | 51 | 85 (75–95) | 529 | 81 (75–92) | 0.53 |
| SBP 24 h | 48 | 140 (130–157) | 500 | 137 (124–150) | 0.015 |
| DBP 24 h | 48 | 80 (70–84) | 500 | 76 (70–82) | 0.47 |
| Glucose | 47 | 7.0 (6.0–8.5) | 518 | 6.5 (5.7–7.8) | 0.071 |
| Aetiological classification | |||||
| Large artery atherosc. | 28/50 | 56.0% | 214/504 | 42.5% | 0.066 |
| Cardioembolic | 9/50 | 18.0% | 131/504 | 26.0% | 0.21 |
| Other or undetermined | 13/50 | 26.0% | 159/504 | 31.5% | 0.42 |
AF: atrial fibrillation; AP: antiplatelet drugs; DBP: diastolic blood pressure; DM: diabetes mellitus; HT: hypertension; nhEND: increase in NIHSS score ≥4 at 24 h with no parenchymal hematoma on follow-up imaging within 22–36 h; NIHSS: NIH Stroke Scale; OAC: oral anticoagulants; OTT: onset-to-treatment time; SBP: systolic blood pressure. All clinical parameters measured at baseline unless specified.
In multivariate analysis (Table 2), any visible arterial occlusion at baseline was the only independent predictor for nhEND, with an adjusted odds ratio (aOR) of 2.5, p < 0.001. When ‘any occlusion’ was substituted in the multivariate model for the detailed ‘specified site of occlusion’ variable, the independent predictors of nhEND remained: ICA-T or ICA + MCA with aOR 10.3, ICA with aOR 4.3, and ACA with aOR 5.9. The remaining variables included in the multivariate model, heart failure, diabetes, glucose (included per the univariate p ≤ 0.10 cut point specified in the ‘Methods’ section), and other occlusion sites did not show significant relationships with nhEND. However, MCA-M1 occlusion (aOR 2.1, p = 0.06), vertebral artery occlusion (aOR 2.3, p = 0.06), heart failure (aOR 1.8 (0.95–3.4), p = 0.07), and diabetes (aOR 1.4 (0.95–2.2), p = 0.08) trended towards significance, while baseline blood glucose did not show a significant association, p = 0.60 (Online Supplement Table 3).
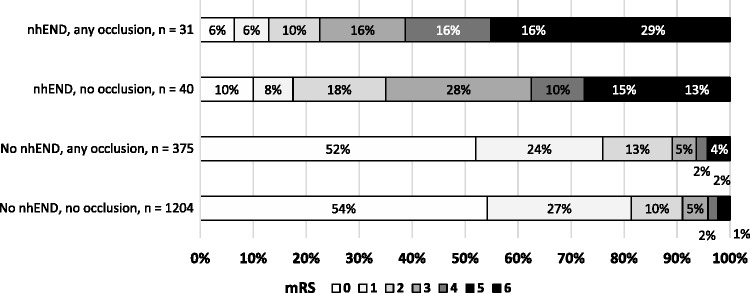
Patients who suffered nhEND after a minor stroke treated with IVT had poor three-month outcomes. Only 23% attained mRS 0–2 in the presence of a baseline occlusion, and 35% among those without an occlusion (Figure 2). Meanwhile, patients who avoided nhEND fared much better, with frequencies of mRS 0–2 at 88 and 92% (with and without occlusion, respectively). In the group with occlusion at baseline, among the 80 patients with poor three-month outcomes (mRS 3–6), 35% had suffered nhEND, while 9% had deterioration associated with PH on follow-up imaging.
Figure 2.
Outcomes per the mRS at three months by arterial occlusion and nhEND status. mRS: modified Rankin Scale; nhEND: non-haemorrhagic early neurological deterioration.
The worst three-month outcomes were seen in the group with ICA-T or ICA-MCA occlusions (mRS 3–6 in 42%), followed by those with occlusion of the ACA (40%), MCA-M1 (20%), basilar artery (19%), vertebral artery (18%), and ICA (17%). Patients with occlusions of M2-MCA, M3-MCA, PCA and those without visible occlusion all had proportions of poor outcomes between 10 and 12% (Online Figure 1).
Discussion
The clinical scenario of an IVT-treated patient with minor stroke symptoms due to an imaging-proven arterial occlusion is not uncommon. In the SITS-ISTR, during the current study period, 21% of patients treated with IVT for acute ischaemic stroke had NIHSS scores 0–5, which is in agreement with data from the Get With the Guidelines registry, reporting 22%.24 In our material, 25% of patients had a visible arterial occlusion on baseline imaging. In previously published cohorts of minor stroke patients treated and untreated with IVT, 14–54% are reported to have a baseline arterial occlusion. The wide variation is explained by differences in definitions of minor stroke and arterial occlusion, treatment status, and other cohort specifics; however, many publications report occlusion frequencies above 30%.5,25–28 In patients with minor stroke due to large artery occlusion, there is no clear evidence on the best course of action beyond IVT therapy, and it is currently unknown whether, and in whom, endovascular recanalisation would be beneficial.
We present a large observational study of patients in this situation, giving estimates of prognosis in relation to the location of arterial occlusion. Patients with minor stroke are at high risk of severe neurological deterioration and poor long-term outcome particularly if the stroke is caused by occlusion of the ICA. It is especially true in the presence of ICA-T and tandem ICA+MCA occlusion, where 30% of patients will deteriorate severely (and not due to SICH) by the second day of hospital stay – a 10-fold increase in the risk of nhEND compared to patients without visible occlusion at baseline. This makes the ‘minor’ in the initial presentation a misnomer. Preventing deterioration in patients with proven arterial occlusion is of paramount importance, as 77% of those who suffer nhEND will be dead or dependent (mRS 3–6) at three months, despite presenting with NIHSS scores 0–5. Put in perspective, these outcomes are worse than in patients treated with IVT for severe stroke (NIHSS score 15–25), where 71% are dead or dependent at three months.23
Regarding the particularly high risk for nhEND in patients with occlusion of the ICA (aOR 4.3) and ICA-T or ICA+MCA (aOR 10.3), our findings are in line with results from previously published studies. In the SAMURAI rt-PA registry, ICA occlusion had an adjusted OR for END of 5.4.6 However, the study investigated END in unselected thrombolysed patients and did not report on minor stroke specifically. One study of patients with minor stroke (NIHSS 0–3) untreated with IVT, also reported a strong association of ICA occlusion with severe END, with aOR 6.5.5 In our study, IVT-treated minor stroke patients with occlusions of the MCA-M1, basilar artery, and ACA faced a lower, but still significantly increased risk of nhEND, with a three- to five-fold increase compared to patients without visible occlusion at baseline. It is important to note that MCA-M1 occlusions, in spite of not quite reaching statistical significance in multivariate analysis (aOR 2.1, 95% CI 0.97–4.4, p = 0.061), had a 9.3% crude rate of deterioration. It is possible that this moderately increased risk can be explained by the fact that these patients present with NIHSS 0–5 in spite of having MCA-M1 occlusion, which could indicate robust collateral circulation or incomplete occlusion with some degree of patency left in the M1 and therefore higher likelihood of effective thrombolysis.29
Our findings on ACA occlusions are difficult to interpret, as patients with ACA occlusions were very few, n = 13 in total, 2% of all visible occlusions and 0.5% of the entire NIHSS 0–5 cohort. Of these, one had a parenchymal haemorrhage and was excluded (per the ‘Methods’ section) and of the remaining 12, two patients had nhEND (∼16.7%). It is not impossible that the two deteriorating patients could have had very proximal A1 occlusions just after the ICA terminus. In such cases, deterioration could have occurred due to retrograde thrombus propagation (build-up) into the terminal ICA and/or M1. However, our data lack the level of detail necessary to elucidate this in further detail.
In minor stroke without arterial occlusion on baseline imaging, nhEND was uncommon, occurring in only 3% of patients, of whom nearly two-thirds had poor three-month outcomes. We have no data on the causes of neurological deterioration in these cases, but in light of the relative rarity of this complication when there is no occlusion, the mechanisms are likely to be heterogeneous, including, among others, recurrent (e.g. cardioembolic) or progressive (e.g. microangiopathic) cerebral ischaemia with a variety of causes.
Due to a lack of data on cerebral haemodynamics and infarct volumes, we are unable to present direct evidence on the mechanisms of nhEND in patients with baseline arterial occlusion. Meanwhile, using clinical data and aetiological stroke classification, we can infer that (1) nhEND could only infrequently have occurred due to early recurrence of cardioembolism, as only 7.5% of deteriorated patients had atrial fibrillation at baseline and 18% of the strokes were cardioembolic, compared to 16.5 and 26%, respectively, in the non-deteriorated group; (2) deterioration due to cerebral oedema within the primary infarct is unlikely among patients with a baseline stroke severity of NIHSS 0–5; (3) systemic hypotension is unlikely to have been a major factor in nhEND, in whom the frequency of congestive heart failure was 11.3% and median 24 h blood pressure was 140/80. We surmise that two likely mechanisms for nhEND in our patients remain: collateral circulation failure10–12 and/or progressive thrombosis,15,30,31 either leading to infarction of initially functional, non-penumbral tissue.32,33 The former would be supported by the low initial symptom severity, which in the presence of a proximal large artery occlusion would indicate good collateral flow to the downstream territory.34 The latter gains support from the somewhat higher proportion of large artery atherosclerosis as cause of stroke among nhEND patients, also reported in previous publications.5,35
There is currently no established therapy to maintain collateral flow. For progressive thrombosis, very early (within hours following IVT) antithrombotic therapy could theoretically prevent nhEND. There is no evidence for benefit of this strategy, however; rather the contrary, as aspirin loading within 24 h from IVT has been shown to increase the risk of SICH.36 Thus, in patients at the highest risk of nhEND, despite low baseline NIHSS scores, it may be reasonable to consider acute treatment with endovascular thrombectomy and/or cervical ICA recanalisation. This conclusion is in line with the current consensus statement on treatment issued by the European Stroke Organisation – Karolinska Stroke Update and endorsed by several European professional societies. It recommends thrombectomy in addition to IVT in patients with large artery occlusion up to 6 h from symptom onset, purposefully omitting the issue of initial symptom severity to allow for an individualised approach to treatment.37 An alternative course of action would be to ensure that patients with occlusions in the most deterioration-prone vessel segments are held under intensive neurological monitoring for at least 24 h from onset at a comprehensive stroke centre to enable rapid endovascular rescue treatment if deterioration should occur. Meanwhile, a number of publications have recently appeared on the topic of primary versus deterioration-driven thrombectomy versus medical therapy alone in minor stroke patients. Although based on small cohorts, these early results indicate that primary intervention may potentially be superior to both deterioration-driven thrombectomy and medical therapy.38–40 This warrants further confirmation, ideally in the setting of at least one multi-centre randomised trial.
In our study, acute vessel imaging in IVT-treated minor stroke patients had a 9% PPV and 97% NPV for nhEND. Despite the initial ‘minor’ presentation, patients who suffer non-haemorrhage-related deterioration have in effect a disease which leads to outcomes as poor, or worse, as in IVT-treated severe stroke: a 77% probability of death or dependence by three months. In patients with the highest risk of nhEND, i.e. those with proximal cerebral artery occlusions, such outcomes may be preventable by acute endovascular treatment. To identify these potentially treatable cases, acute vessel imaging should preferably be performed routinely in all patients with acute ischaemic stroke regardless of symptom severity. As an additional benefit, acute imaging allows earlier decision-making regarding carotid endarterectomy in patients who harbour significant carotid stenosis, a more rapid and better informed individualisation of aetiological workup and secondary preventive treatment. The corollary also applies: the NPV of 97% indicates that minor stroke in absence of vessel occlusion in the majority of cases has a benign prognosis, which may guide decisions on early neurological monitoring and discharge planning.
The results of this study are subject to limitations inherent to observational design. Similar to the situation in other large-scale registries, the data in the SITS-ISTR, while having the benefit of being representative of a wide range of practice settings and countries, are collected with no independent adjudication. Of 6860 patients with minor stroke and IVT treatment collected in our database between 2012 and 2016, 2620 (38%) had relevant baseline vessel imaging performed. This is reflective of practice in many centres and countries, where CTA or MRA is not routinely used in minor stroke, and in others, is used selectively. These numbers do raise the question of potential patient selection bias. However, we report 25% of patients with visible occlusions, and many recent publications have reported proportions above 30%.5,25–28 This would limit the likelihood that patients in our material were selected for vessel imaging based on any unregistered factors increasing their likelihood of harbouring an occlusion. In order to analyse nhEND in a cohort treated with only IVT, from our initial population of 2620 patients, we excluded 67 (2.6%) patients who had undergone endovascular therapy (Online Table 2). In particular, since 10 of 39 patients with basilar artery occlusion were excluded for this reason, our results in this group need to be interpreted with caution. The exclusion may have been a source of selection bias potentially lowering the proportion of nhEND in our results, since endovascular therapy in some of the excluded patients could have been performed because of END, or if done in the hyperacute phase, might have prevented deterioration. An important limiting factor for the interpretation of our results on extracranial ICA occlusion is our inability to discriminate between complete occlusion and significant stenosis of the vessel based on the SITS database structure. This limits our conclusions on ICA occlusion in minor stroke to a recommendation on close neurological monitoring and need for further studies on the prevention of clinical deterioration due to ICA pathology in the ultra-acute phase of stroke. A further limitation requiring mention is the relatively high proportion of patients without available three-month outcome data (31%). Importantly, this does not influence the interpretation of results pertaining to our primary aim: to study early deterioration in relation to occlusion sites in minor stroke. Meanwhile, it warrants caution in the interpretation of our secondary analyses of long-term functional outcome. While acknowledging this, one must still note that patients with END have been extensively shown in prior publications to have a very poor prognosis,14,15 which we were able to confirm even in the presence of the mentioned limitations.
Conclusion
Patients with apparently minor stroke associated with occlusion of the ICA, with or without tandem MCA involvement, are at high risk of disabling deterioration, despite IVT treatment. Acute vessel imaging contributes usefully even in minor stroke patients to identify and consider acute endovascular treatment or intensive neurological monitoring at a neuroendovascular centre, for those who may be at high risk of potentially preventable deterioration. A randomised controlled trial of endovascular treatment versus best medical management including thrombolysis in patients with minor stroke is urgently needed.
Supplementary Material
Acknowledgements
We thank all SITS-ISTR investigators and their centres for their participation. We also pass on our thanks to all patients who participated in SITS-ISTR. The SITS registry is developed, maintained, and upgraded by Zitelab, Copenhagen, Denmark, in close collaboration with SITS.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MVM is the International Network and Research Executive of SITS International, which receives a grant from Boehringer Ingelheim for the SITS-ISTR. KRL has received fees and expenses from Boehringer Ingelheim for lectures and for serving on data monitoring committees. GAF has received lecture fees from Boehringer Ingelheim. CC, TM, MB, LS, and VS have no conflicting interests. SF has received a lecture fee from Boehringer Ingelheim. DT is a member of an Advisory Board (regarding Dabigatran) and has received speaker honoraria from Boehringer Ingelheim. NW is the Chairman of SITS International, which receives a grant from Boehringer Ingelheim for the SITS-ISTR. He has received fees and expenses for lectures and consultancies from Boehringer Ingelheim. NA is the Vice Chairman of SITS International, which receives a grant from Boehringer Ingelheim for the SITS-ISTR.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: SITS is financed directly and indirectly by grants from Karolinska Institutet, Stockholm County Council, the Swedish Heart-Lung Foundation, the Swedish Order of St John, Friends of Karolinska Institutet, and private donors, as well as from an unrestricted sponsorship from Boehringer-Ingelheim. SITS has previously received grants from the European Union Framework 7, the European Union Public Health Authority, and Ferrer Internacional. SITS is currently conducting studies supported by Boehringer-Ingelheim and EVER Pharma, as well as in collaboration with Karolinska Institutet, supported by Stryker, Covidien, and Phenox.
MVM is supported by the Stockholm County Council (clinical postdoctoral appointment).
CC was supported by the Stockholm County Council (combined clinical residency and PhD training program).
NW is supported by grants provided by the Stockholm County Council.
NA is supported by grants provided by the Stockholm County Council and the Swedish Heart-Lung Foundation.
No funding sources had part in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Informed consent
Ethics approval and patient consent for participation in the SITS-ISTR were obtained in countries that required this; other countries approved the register for anonymised audit.
Ethical approval
Ethics approval was obtained from the Stockholm Regional Ethics Committee for this project as part of the SITS-MOST II study framework.
Guarantor
MVM and NA had full access to all data in this study and hold final responsibility for the preparation and content of this manuscript, and its submission for publication.
Contributorship
The study protocol was drafted by MVM, NA, CC, TM, and NW. It was reviewed and commented upon by members of the SITS Scientific Committee (KRL, DT, GAF, NA, NW). MB, SF, TM, LS, VS, DT, KRL, and GAF were coordinators of data collection at major recruiting centres or countries. Data analysis was carried out by MVM with input by CC and NA. MVM wrote the final draft of the manuscript. All authors reviewed and made important intellectual contributions to the final draft.
References
- 1.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014; 384: 1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kharitonova T, Ahmed N, Thoren M, et al. Hyperdense middle cerebral artery sign on admission CT scan – prognostic significance for ischaemic stroke patients treated with intravenous thrombolysis in the safe implementation of thrombolysis in stroke international stroke thrombolysis register. Cerebrovasc Dis 2009; 27: 51–59. [DOI] [PubMed] [Google Scholar]
- 3.Cooray C, Fekete K, Mikulik R, et al. Threshold for NIH stroke scale in predicting vessel occlusion and functional outcome after stroke thrombolysis. Int J Stroke 2015; 10: 822–829. [DOI] [PubMed] [Google Scholar]
- 4.Heldner MR, Jung S, Zubler C, et al. Outcome of patients with occlusions of the internal carotid artery or the main stem of the middle cerebral artery with NIHSS score of less than 5: comparison between thrombolysed and non-thrombolysed patients. J Neurol Neurosurg Psychiatry 2015; 86: 755–760. [DOI] [PubMed] [Google Scholar]
- 5.Kim JT, Park MS, Chang J, et al. Proximal arterial occlusion in acute ischemic stroke with low NIHSS scores should not be considered as mild stroke. PLoS One 2013; 8: e70996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mori M, Naganuma M, Okada Y, et al. Early neurological deterioration within 24 h after intravenous rt-PA therapy for stroke patients: the stroke acute management with urgent risk factor assessment and improvement rt-PA registry. Cerebrovasc Dis 2012; 34: 140–146. [DOI] [PubMed] [Google Scholar]
- 7.Rajajee V, Kidwell C, Starkman S, et al. Early MRI and outcomes of untreated patients with mild or improving ischemic stroke. Neurology 2006; 67: 980–984. [DOI] [PubMed] [Google Scholar]
- 8.Dubuc V, Singh D, Modi J, et al. TIA and minor stroke patients with intracranial occlusions in both proximal and distal vessels are most at risk for symptom progression. Cerebrovasc Dis 2014; 38: 389–390. [DOI] [PubMed] [Google Scholar]
- 9.Maas MB, Lev MH, Ay H, et al. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke 2009; 40: 3001–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tisserand M, Seners P, Turc G, et al. Mechanisms of unexplained neurological deterioration after intravenous thrombolysis. Stroke 2014; 45: 3527–3534. [DOI] [PubMed] [Google Scholar]
- 11.Campbell BC, Christensen S, Tress BM, et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab 2013; 33: 1168–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell BC, Donnan GA, Davis SM. Vessel occlusion, penumbra, and reperfusion – translating theory to practice. Front Neurol 2014; 5: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seners P, Hurford R, Tisserand M, et al. Is unexplained early neurological deterioration after intravenous thrombolysis associated with thrombus extension? Stroke 2017; 48: 348–352. [DOI] [PubMed] [Google Scholar]
- 14.Khatri P, Conaway MR, Johnston KC. Ninety-day outcome rates of a prospective cohort of consecutive patients with mild ischemic stroke. Stroke 2012; 43: 560–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seners P, Turc G, Oppenheim C, et al. Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: a systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry 2015; 86: 87–94. [DOI] [PubMed] [Google Scholar]
- 16.Coutts SB, Modi J, Patel SK, et al. What causes disability after transient ischemic attack and minor stroke?: results from the CT and MRI in the Triage of TIA and minor Cerebrovascular Events to Identify High Risk Patients (CATCH) Study. Stroke 2012; 43: 3018–3022. [DOI] [PubMed] [Google Scholar]
- 17.Coutts SB, Modi J, Patel SK, et al. CT/CT angiography and MRI findings predict recurrent stroke after transient ischemic attack and minor stroke: results of the prospective CATCH study. Stroke 2012; 43: 1013–1017. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues FB, Neves JB, Caldeira D, et al. Endovascular treatment versus medical care alone for ischaemic stroke: systematic review and meta-analysis. BMJ 2016; 353: i1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powers WJ, Derdeyn CP, Biller J, et al. 2015 American heart association/American stroke association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015; 46: 3020–3035. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed N, Wahlgren N, Grond M, et al. Implementation and outcome of thrombolysis with alteplase 3–4.5 h after an acute stroke: an updated analysis from SITS-ISTR. Lancet Neurol 2010; 9: 866–874. [DOI] [PubMed] [Google Scholar]
- 21.Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 2007; 369: 275–282. [DOI] [PubMed] [Google Scholar]
- 22.Mazya MV, Ahmed N, Ford GA, et al. Remote or extraischemic intracerebral hemorrhage – an uncommon complication of stroke thrombolysis: results from the safe implementation of treatments in stroke-international stroke thrombolysis register. Stroke 2014; 45: 1657–1663. [DOI] [PubMed] [Google Scholar]
- 23.Mazya MV, Lees KR, Collas D, et al. IV thrombolysis in very severe and severe ischemic stroke: results from the SITS-ISTR registry. Neurology 2015; 85: 2098–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romano JG, Smith EE, Liang L, et al. Outcomes in mild acute ischemic stroke treated with intravenous thrombolysis: a retrospective analysis of the get with the guidelines-stroke registry. JAMA Neurol 2015; 72: 423–431. [DOI] [PubMed] [Google Scholar]
- 25.Kohrmann M, Nowe T, Huttner HB, et al. Safety and outcome after thrombolysis in stroke patients with mild symptoms. Cerebrovasc Dis 2009; 27: 160–166. [DOI] [PubMed] [Google Scholar]
- 26.Zhu W, Churilov L, Campbell BC, et al. Does large vessel occlusion affect clinical outcome in stroke with mild neurologic deficits after intravenous thrombolysis? J Stroke Cerebrovasc Dis 2014; 23: 2888–2893. [DOI] [PubMed] [Google Scholar]
- 27.Laurencin C, Philippeau F, Blanc-Lasserre K, et al. Thrombolysis for acute minor stroke: outcome and barriers to management. Results from the RESUVAL stroke network. Cerebrovasc Dis 2015; 40: 3–9. [DOI] [PubMed] [Google Scholar]
- 28.Choi JC, Jang MU, Kang K, et al. Comparative effectiveness of standard care with IV thrombolysis versus without IV thrombolysis for mild ischemic stroke. J Am Heart Assoc 2015; 4: e001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahn SH, d’Esterre CD, Qazi EM, et al. Occult anterograde flow is an under-recognized but crucial predictor of early recanalization with intravenous tissue-type plasminogen activator. Stroke 2015; 46: 968–975. [DOI] [PubMed] [Google Scholar]
- 30.Seners P, Hurford R, Tisserand M, et al. Is unexplained early neurological deterioration after intravenous thrombolysis associated with thrombus extension? Stroke 2017; 48: 348. [DOI] [PubMed] [Google Scholar]
- 31.Seners P, Turc G, Tisserand M, et al. Unexplained early neurological deterioration after intravenous thrombolysis: incidence, predictors, and associated factors. Stroke 2014; 45: 2004–2009. [DOI] [PubMed] [Google Scholar]
- 32.Alawneh JA, Moustafa RR, Baron JC. Hemodynamic factors and perfusion abnormalities in early neurological deterioration. Stroke 2009; 40: e443–e450. [DOI] [PubMed] [Google Scholar]
- 33.Alawneh JA, Jones PS, Mikkelsen IK, et al. Infarction of ‘non-core-non-penumbral’ tissue after stroke: multivariate modelling of clinical impact. Brain 2011; 134: 1765–1776. [DOI] [PubMed] [Google Scholar]
- 34.Sheth SA, Sanossian N, Hao Q, et al. Collateral flow as causative of good outcomes in endovascular stroke therapy. J Neurointerv Surg 2016; 8: 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simonsen CZ, Schmitz ML, Madsen MH, et al. Early neurological deterioration after thrombolysis: clinical and imaging predictors. Int J Stroke 2016; 11: 776–782. [DOI] [PubMed]
- 36.Zinkstok SM, Roos YB. ARTIS-Investigators. Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: a randomised controlled trial. Lancet 2012; 380: 731–737. [DOI] [PubMed] [Google Scholar]
- 37.Wahlgren N, Moreira T, Michel P, et al. Mechanical thrombectomy in acute ischemic stroke: consensus statement by ESO-Karolinska Stroke Update 2014/2015, supported by ESO, ESMINT, ESNR and EAN. Int J Stroke 2016; 11: 134–147. [DOI] [PubMed] [Google Scholar]
- 38.Haussen DC, Bouslama M, Grossberg JA, et al. Too good to intervene? Thrombectomy for large vessel occlusion strokes with minimal symptoms: an intention-to-treat analysis. J Neurointerv Surg 2017; 9: 917–921. [DOI] [PubMed] [Google Scholar]
- 39.Haussen DC, Lima FO, Bouslama M, et al. Thrombectomy versus medical management for large vessel occlusion strokes with minimal symptoms: an analysis from STOPStroke and GESTOR cohorts. J Neurointerv Surg. Epub August 2017. doi: 10.1136/neurintsurg-2017-013243. [DOI] [PubMed]
- 40.Messer MP, Schonenberger S, Mohlenbruch MA, et al. Minor stroke syndromes in large-vessel occlusions: mechanical thrombectomy or thrombolysis only? Am J Neuroradiol 2017; 38: 1177–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.