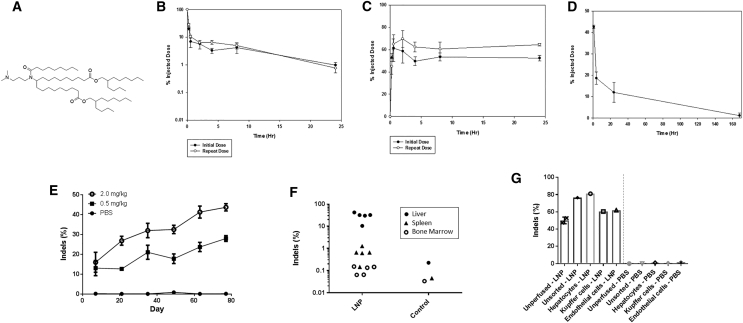
Figure 1.
Characterization of In Vivo LNP Delivery
(A) Structure of ionizable lipid used in LNP formulation. Pharmacokinetics of lipid in plasma (B) and biodistribution to liver (C) after single and repeat intravenous administration of LNPs in mice (n = 5). (D) Elimination of lead ionizable lipid from liver following intravenous injection in mice (n = 5). (E) Genome-editing results from mouse bulk liver tissue following six repeat intravenous administrations of ionizable lipid-containing LNPs comprising mRNAs encoding ZFNs 48641 and 31523 (n = 4–6 per data point) (p < 0.0001, R2 = 0.5557, F = 32.52, degrees of freedom = 26 for 0.5 mg/kg from first to sixth dose; p < 0.0001, R2 = 0.6996, F = 55.9, degrees of freedom = 24 for 2.0 mg/kg from first to sixth dose). (F) Biodistribution of genome modification following a single 2-mg/kg administration of lead ionizable lipid-containing LNP comprising mRNAs encoding ZFNs 48641 and 31523 (n = 5 per LNP group organ). Note background detection level of indels of 0.1%–0.2% (p = 0.00054 comparing liver versus spleen; p = 0.00048 comparing liver versus bone marrow). (G) Genome modification of mice from (F), which were either sacrificed and unmanipulated prior to liver harvest (unperfused) or perfused through the hepatic portal vein with buffered saline prior to liver harvest to remove blood cells within the liver (unsorted) as well as to prepare the liver for perfusion with a collagenase solution. A fraction of the perfused livers was also FACS-sorted into individual liver cell populations (n = 1–2 per group).