Abstract
Purpose
The use of platelet-rich plasma (PRP) for the treatment of nonhealing ulcers is a relatively new technique. Although it seems to result in a satisfying level of healing and low complication rates, data regarding its effectiveness remain sparse. This study aims to evaluate the potential therapeutic effects of PRP on chronic nonhealing ulcers.
Materials and Methods
This was a prospectively designed study comparing outcomes between patients treated with PRP (Group A, n=15) and patients treated conventionally (Group B, n=12) for different types of nonhealing ulcers. In Group A, PRP was produced from the patients’ own peripheral blood samples and was applied on the ulcer once every week. In Group B, patients were treated conventionally, without applying PRP. The total treatment period was 5 weeks.
Results
Both groups were similar regarding age, sex, comorbidities, and time of treatment. In Group A, patients showed a nonsignificant reduction of 4.5 mm2 in ulcer area (P=0.190) and a reduction of more than 1 mm in depth (P=0.0006), while Group B showed an increase of 108±80.5 mm2 in ulcer area after 5 weeks (P=0.016). The healing rate (HR) in Group A was stable and positive throughout the treatment period, while HR in Group B was initially negative but became positive after the 3rd week.
Conclusion
PRP application once a week promotes the healing of chronic ulcers. It improves the ulcer’s depth and HR, although its effect on ulcer area seems to be nonsignificant. However, larger comparative series are still needed to confirm these findings.
Keywords: Platelet-rich plasma, Ulcer, Regeneration
INTRODUCTION
The application of platelet-rich plasma (PRP) for the treatment of chronic ulcers is a relatively novel technique. This method has been successfully utilized in different specialties, such as orthopedics, maxillofacial and dental surgery, and general and plastic surgery [1–5]. Moreover, PRP has a relatively small number of contraindications and has limited side effects [6].
Briefly, the mechanisms of action of PRP are as follows: The platelets participate in clot formation within the coagulation. After the blood vessel injury, the collagen originating from the surrounding connective tissue flows directly over the bloodstream, and together with other agents, it activates platelet aggregation and activation [7]. After clot formation, the platelets degranulate and release the following growth factors: platelet-derived growth factor (PDGF), insulin-like growth factor-1, epidermal growth factor, transforming growth factor-β, vascular endothelial growth factor, and fibroblast growth factors [8]. Finally, PRP has been proven to enhance the localized healing potential of growth factors by promoting the growth of granulomatous tissue; the activation of mechanisms leading to collagen production; the aggregation of fibroblasts, macrophages, and other cells; and the development of new epithelium [9]. However, although the mechanism of PRP action is clear, the clinical benefits of this treatment need to be further verified.
Therefore, this study aimed to evaluate the potential therapeutic effects of PRP in patients with different types of chronic ulcers to produce useful conclusions for everyday clinical practice.
MATERIALS AND METHODS
This was a prospectively designed study that evaluated the effect of PRP in patients with different types of ulcers. The Ethical Committee of Hippocration General Hospital, Athens, Greece has approved the study (approval no. 22/6-6-2011). All patients were enrolled during a 3-year period following referral for surgical treatment of a pressure ulcer or a nonhealing lower limb ulcer, and they remained inpatients at the time of referral and during treatment. Inclusion and exclusion criteria for all patients are presented in Table 1.
Table 1.
Inclusion and exclusion criteria in this study
Inclusion criteria
|
Exclusion criteria
|
Regarding allocation, all patients were randomly divided into two groups. The intervention group (Group A) received treatment that included PRP application, conventional debridement, and dressing coverage. Additionally, the control group (Group B) underwent the same conventional debridement and dressing coverage, but without any PRP application. In order to avoid any allocation bias, and as the patients were not treated at the same time but upon admission, the first half of the patients were allocated to the intervention group, and the second half of the patients were allocated to the control group. Only the patients who received treatment for at least 2 weeks were included in subsequent statistical analysis. All patients who failed to be followed-up for the required period of time were excluded.
In all patients, medical history was carefully recorded, all underlying medical issues were addressed, and clinical evaluation of the ulcer was performed. Moreover, all patients signed the informed consent. Measurements of all dimensions (width, length, and depth) were recorded for all ulcers. When necessary, surgical debridement was performed. The ulcer was cleaned with sterile saline, and biopsies from the ulcer edges and ulcer bed were taken in order to exclude any malignancy. In Group A, PRP clot or injection was applied on the ulcer, and the ulcer was covered with sterile paraffin gauze and sterile gauze or pressure wound dressing (subject to availability). The dressing was kept in place for 2 days, and after that, conventional dressing was applied on alternate days. The PRP treatment was repeated once every week. The patients in Group B underwent debridement only, and then conventional dressing was applied on alternate days. The debridement was repeated every week if necessary.
PRP preparation and application was conducted as follows: The method is based on the separation of platelets from other blood components, such as red blood cells by centrifugation. Blood sample is taken from the patient and it is then centrifuged, and PRP and platelet-poor plasma (PPP) are produced. The ulcer can be sprayed or injected with PRP, or a clot can be produced and applied to the ulcer. A variety of commercially marketed PRP preparation systems are available. We used the following two different systems: gravitational platelet separation (GPS) II system (Biomet Biologics, Inc., Warsaw, IN, USA, distributed by Biomet Hellas) and RegenKit (Regen Lab, Mollens, Switzerland, distributed by Arthrosis S.A Greece) [10].
GPS II system requires 27–108 mL of the patient’s blood, which is added to anticoagulant citrate dextrose solution or Solution A (ACD-A). After centrifugation at 3,200 revolutions per minute for 15 minutes, it produces 3–12 mL of platelet concentrate with a platelet count 3- to 8-fold the peripheral blood concentration. The platelets can then be activated with calcium solution (Biomet Biologics, Inc.). Regen PRP uses 23 mL of whole blood added to ACD-A and centrifuged at 1,500 g (gravity force) for 6 minutes. It produces 8 mL of PRP with platelet count 2- to 4-fold the peripheral blood concentration and 3-mL thrombin for platelet activation (Regen Lab). PRP is then applied to the ulcer either as an injection to the ulcer edges or as spray for small, shallow or activated ulcers, and the produced clot is applied into the ulcer cavity for deep ulcers.
For all patients, the main epidemiologic data were reported. The main outcome was ulcer healing within 1 to 5 weeks. The healing in both groups was recorded by measuring the ulcer’s three dimensions (length, width, and depth). We calculated the area (mm2) of the ulcer based on the assumption that every ulcer has elliptic shape. The difference of the mean areas between 2 consecutive weeks was used as a comparison parameter and was referred as healing rate (HR).
Statistical analysis was performed using the StatsDirect statistical software ver. 2.8.0 (StatsDirect Ltd., Altrincham, Cheshire, UK). Comparisons between groups were performed using the independent t-test for continuous variables if the distribution was normal and Mann-Whitney U-test if the distribution was not normal. Chi-squared and Fisher’s exact tests were used for categorical variables as appropriate. Continuous data are presented as means±standard deviation. A P-value of <0.05 was considered statistically significant. Multivariate logistic regression was used to identify the independent associations between various risk factors and ulcer healing.
All human studies have been performed in accordance with the Declaration of Helsinki.
RESULTS
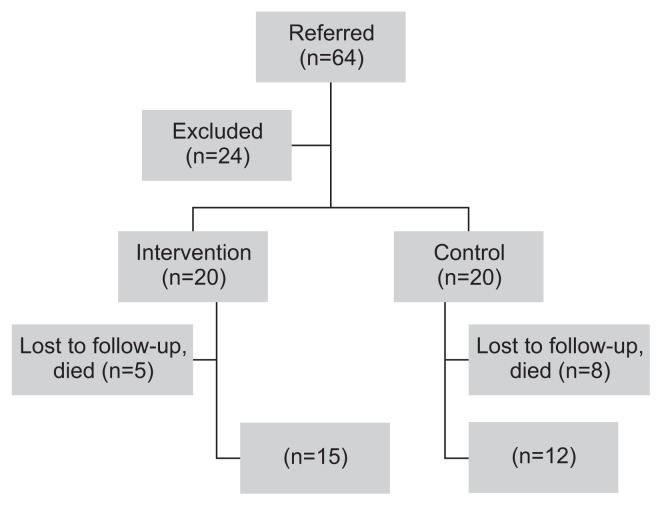
Overall, 64 patients were referred for treatment, and 24 were excluded in total before allocation. The remaining 40 patients were allocated into Group A (n=20) and Group B (n=20). Five patients from Group A and eight from Group B were subsequently not included in the statistical analysis for various reasons (lost to follow-up, deterioration, death, aforementioned exclusion criteria). Finally, 15 patients from Group A and 12 patients from Group B were included in the analysis (Fig. 1).
Fig. 1.
Consort diagram.
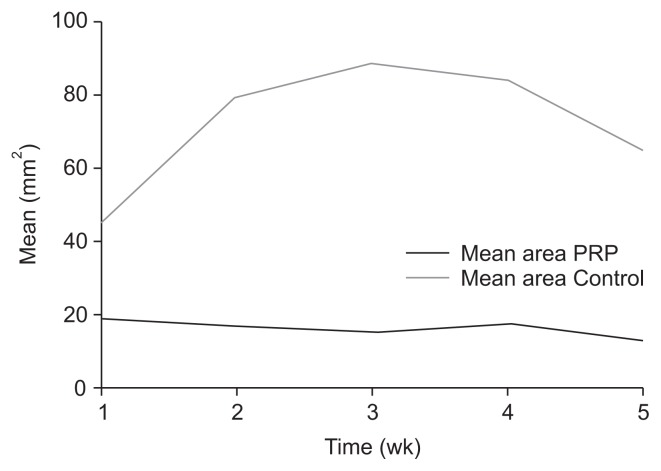
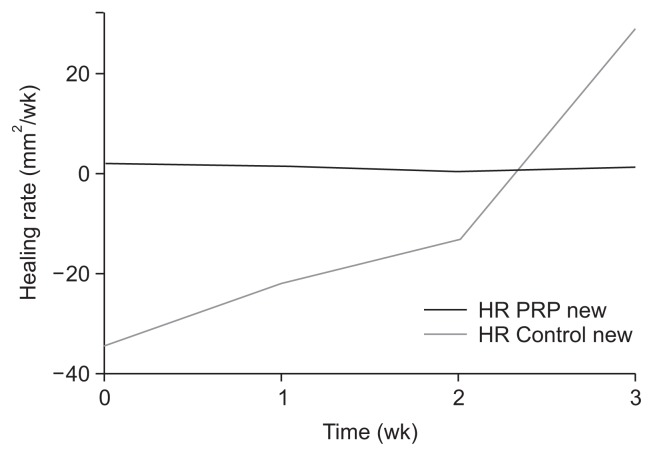
Both groups were similar regarding age, sex, and major comorbidities (Table 2). Additionally, hypoalbuminemia was not different between the two groups. Group A showed steady reduction in ulcer area and depth, while Group B initially showed an increase in ulcer size that was followed by a decreased ulcer size after week 3 (Fig. 2). Overall, Group B showed a mean increase in the ulcer area of 108 mm2 after 5 weeks (P=0.016), while Group A showed a mean reduction of 4.5 mm2, which was not significant (P=0.190) (Table 3). Mean depth of the ulcer was also lower after 5 weeks in Group A (P=0.0006), although ulcer depth showed a small reduction in Group B after 5 weeks, which was also not significant (P=0.104). Two patients in the treatment group achieved complete epithelialization by week 8, but none in the control group reached healing. HR in Group A was stable and positive throughout the recorded treatment period, while the control group initially showed negative HR that became positive after the 3rd week of treatment (Fig. 3). Finally, multivariate analysis did not identify any of the reported variables as a predictor for ulcer healing.
Table 2.
Demographics
| Variable | Control group (n=12) | Intervention group (n=15) | P-value |
|---|---|---|---|
| Age (y) | 67.40±14.50 | 67.90±16.60 | P=0.900 |
| Sex | P=0.100 | ||
| Male | 4 (33.3) | 9 (60.0) | |
| Female | 8 (66.7) | 6 (40.0) | |
| Diabetes mellitus | 1 | 3 | P=0.605 |
| Coronary disease | 0 | 1 | P=1.000 |
| COPD | 1 | 1 | P=1.000 |
| Cerebrovascular diseasea | 8 | 4 | P=0.057 |
| Hypoalbuminemia | 4 | 4 | P=1.000 |
| Mean treatment length (wk) | 4.17±1.70 | 3.93±2.00 | P=0.633 |
| Mean area on admission (mm2) | 45.13±52.70 | 18.82±12.50 | P=0.196 |
| Site of ulcer | |||
| Coccyx | 12 | 8 | P=0.056 |
| Stump | 0 | 4 | |
| Foot | 0 | 2 | |
| Heel | 0 | 1 | |
| Total | 2 | 15 | |
Values are presented as mean±standard deviation, number (%), or number only.
COPD, chronic obstructive pulmonary disease.
Stroke, dementia, Parkinson disease or intracranial hemorrhage.
Fig. 2.
Diagram of mean ulcer area (control group vs. treatment group). Mean area platelet-rich plasma (PRP), treatment group; Mean area Control, control group.
Table 3.
Mean area and depth of ulcer in each group during the follow-up period
| Outcome | Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 |
|---|---|---|---|---|---|---|
| Area (mm2) | ||||||
| Control | 45.13±52.70 | 79.75±96.00 | 88.67±104.00 | 84.19±105.50 | 65.02±81.20 | 153.08±133.20 P=0.016 (vs. week 0) |
| Treatment | 18.82±12.50 | 16.82±12.30 | 15.50±11.60 | 17.61±6.30 | 13.00±9.50 | 14.46±1.40 P=0.190 (vs. week 0) |
| Depth (mm) | ||||||
| Control | 1.85±1.72 | 2.56±1.89 | 3.06±2.14 | 2.18±1.09 | 1.76±1.16 | 1.00±0.23 P=0.104 (vs. week 0) |
| Treatment | 1.28±1.15 | 0.98±0.92 | 0.91±0.69 | 0.33±0.45 | 0.50±0.33 | 0.13±0.01 P=0.0006 (vs. week 0) |
Values are presented as mean±standard deviation.
Fig. 3.
Diagram of HR (control group vs. treatment group). Healing rate (HR) PRP, treatment group; HR Control, control group.
DISCUSSION
This study has found that the application of PRP on previously chronic nonhealing ulcers has a beneficial effect as far as ulcer depth and HR are concerned.
Pressure ulcers pose a significant problem for the treatment of bed-bound patients, especially in the elderly, and they have a tremendous effect on their quality of life. Prevalence rates remain unacceptably high, ranging between 6% and 20% [11,12]. According to the recent National Institute of Care and Health Excellence Pressure Ulcers Quality standards, the National Reporting and Learning System (UK) found that pressure ulcers had the largest proportion of patient safety incidents in 2011–2012, accounting for 19% of all reports. Diabetic foot ulcer is another type of chronic nonhealing ulcer. The most important factors underlying the development of foot ulcers are peripheral sensory neuropathy, foot deformities related to motor neuropathy, minor foot trauma, and peripheral arterial disease [13]. Infection, tissue hypoxia, necrosis, exudate, and excess levels of inflammatory cytokines are the major risk factors that prolong the healing of chronic ulcers [14]. Additionally, growth factors, cytokines, proteases, and cellular and extracellular elements play an important role in the different phases of the healing process, and alterations in one or more of these components may cause impaired healing [15].
Our study has shown that the use of PRP promotes the healing of chronic ulcers, although we could not evaluate its effect on each type of ulcers separately due to the small number of patients. The efficiency of this method is based on the local and continuous delivery of a wide range of PDGFs and proteins that mimic the natural healing process [16,17]. Treatment with PRP seems to limit the noxious effect of deep tissue injury, preventing the spread of tissue necrosis [18]. According to a recent study by Oomens et al. [19], a pressure-induced injury to muscle fibers, possibly at the cytoskeleton level, could in part be responsible for the development of pressure ulcers. Based on the findings of our study, we can assume that the growth factors are involved in the prevention of spread or even repair of such injury, and molecular mechanisms seem to be even more complex. However, data are still sparse, and more studies are required to shed light on those processes.
Our results indicate that PRP has the most pronounced effect on the depth of the ulcer concurring with other authors. Experimental models have provided substantial evidence to support that platelet-rich fibrin matrix – a variant preparation with similar properties – induces endothelial cell proliferation that may suggest an explanation for this type of effect [20]. Additionally, we have found a significantly higher HR in patients treated with PRP. Saad-Setta et al. [21] have compared PRP with PPP in patients with chronic diabetic foot ulcers and also showed that healing with PRP was significantly faster. It seems that PRP promotes cell migration and proliferation, causing a rapid healing of chronic ulcers that could justify this effect. Finally, we have applied PRP only once a week in our study, although more authors seem to prefer a twice-a-week strategy [22,23].
According to the literature, PRP may improve the healing of foot ulcers associated with diabetes [24], but this conclusion is based on low-quality evidence from small randomized controlled trials [25]. However, our study found that PRP was not associated with diabetes mellitus or other major outcomes. Regarding other confounding factors, we have also found that there is no correlation between hypoalbuminemia and major outcomes of PRP. This concurs with other studies as well. Ramos-Torrecillas et al. [26] have found no association between the blood levels of albumin or total proteins and the PRP healing process, which is also consistent with the finding of de Leon et al. [27], that is, there is no relationship between serum albumin or hemoglobin levels and the effects of Plasma Rich in Growth Factors on chronic ulcers.
Finally, the limitations of our study include the small number of patients included in the study, although statistical significance could be found. Due to study planning, blinded randomization was not feasible, as neither the healthcare provider nor the patients could be blinded. However, the randomization problem was addressed by allocating the first 20 patients to the treatment group and the last 20 patients to the control group, thus avoiding selection bias. Regarding the confounding factors, ulcer area size and depth could affect initial treatment decisions and increase potential bias, especially in correlation with ulcer’s location. Therefore, a blind randomization would be optimal for safer results.
CONCLUSION
In conclusion, PRP promotes the healing of previously chronic nonhealing ulcers. PRP seems to have a beneficial effect on ulcer depth and HR. However, larger comparative studies are needed to verify these results.
Footnotes
CONFLICTS OF INTEREST
The authors have nothing to disclose.
REFERENCES
- 1.Mehta S, Watson JT. Platelet rich concentrate: basic science and current clinical applications. J Orthop Trauma. 2008;22:432–438. doi: 10.1097/BOT.0b013e31817e793f. [DOI] [PubMed] [Google Scholar]
- 2.Albanese A, Licata ME, Polizzi B, Campisi G. Platelet-rich plasma (PRP) in dental and oral surgery: from the wound healing to bone regeneration. Immun Ageing. 2013;10:23. doi: 10.1186/1742-4933-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spyridakis M, Christodoulidis G, Chatzitheofilou C, Symeonidis D, Tepetes K. The role of the platelet-rich plasma in accelerating the wound-healing process and recovery in patients being operated for pilonidal sinus disease: preliminary results. World J Surg. 2009;33:1764–1769. doi: 10.1007/s00268-009-0046-y. [DOI] [PubMed] [Google Scholar]
- 4.Bhanot S, Alex JC. Current applications of platelet gels in facial plastic surgery. Facial Plast Surg. 2002;18:27–33. doi: 10.1055/s-2002-19824. [DOI] [PubMed] [Google Scholar]
- 5.Vick VL, Holds JB, Hartstein ME, Rich RM, Davidson BR. Use of autologous platelet concentrate in blepharoplasty surgery. Ophthalmic Plast Reconstr Surg. 2006;22:102–104. doi: 10.1097/01.iop.0000202092.73888.4c. [DOI] [PubMed] [Google Scholar]
- 6.Harmon K, Hanson R, Bowen J, Greenberg S, Magaziner E, Vandenbosch J, et al. Guidelines for the use of platelet rich plasma. Int Cell Med Soc. 2013;41:356–364. [Google Scholar]
- 7.Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16:1043–1054. doi: 10.1097/01.scs.0000186454.07097.bf. [DOI] [PubMed] [Google Scholar]
- 8.Everts PA, Knape JT, Weibrich G, Schönberger JP, Hoffmann J, Overdevest EP, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38:174–187. [PMC free article] [PubMed] [Google Scholar]
- 9.McAleer JP, Kaplan E, Persich G. Efficacy of concentrated autologous platelet-derived growth factors in chronic lower-extremity wounds. J Am Podiatr Med Assoc. 2006;96:482–488. doi: 10.7547/0960482. [DOI] [PubMed] [Google Scholar]
- 10.Degen RM, Bernard JA, Oliver KS, Dines JS. Commercial separation systems designed for preparation of platelet-rich plasma yield differences in cellular composition. HSS J. 2017;13:75–80. doi: 10.1007/s11420-016-9519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meehan M. Multisite pressure ulcer prevalence survey. Decubitus. 1990;3:14–17. doi: 10.1097/00129334-199011000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Tubaishat A, Papanikolaou P, Anthony D, Habiballah L. Pressure ulcers prevalence in the acute care setting: a systematic review, 2000–2015. Clin Nurs Res. 2018;27:643–659. doi: 10.1177/1054773817705541. [DOI] [PubMed] [Google Scholar]
- 13.Van Netten JJ, Bakker K, Apelqvist J, Lipsky BA, Schaper NC. The 2015 guidance of the International Working Group on the Diabetic Foot. EWMA J. 2016;16:11–14. [Google Scholar]
- 14.Vanwijck R. [Surgical biolog y of wound healing]. Bull Mem Acad R Med Belg. 2001;156:175–184. discussion 185. French. [PubMed] [Google Scholar]
- 15.Enoch S, Leaper DJ. Basic science of wound healing. Surg. 2008;26:31–37. [Google Scholar]
- 16.Anitua E, Sánchez M, Nurden AT, Nurden P, Orive G, Andía I. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006;24:227–234. doi: 10.1016/j.tibtech.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Anitua E, Alkhraisat MH, Orive G. Perspectives and challenges in regenerative medicine using plasma rich in growth factors. J Control Release. 2012;157:29–38. doi: 10.1016/j.jconrel.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528–1542. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- 19.Oomens CW, Bader DL, Loerakker S, Baaijens F. Pressure induced deep tissue injury explained. Ann Biomed Eng. 2015;43:297–305. doi: 10.1007/s10439-014-1202-6. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz S, Aksoy E, Doganci S, Yalcinkaya A, Diken AI, Cagli K. Autologous platelet-rich plasma in treatment of chronic venous leg ulcers: a prospective case series. Vascular. 2015;23:580–585. doi: 10.1177/1708538114563824. [DOI] [PubMed] [Google Scholar]
- 21.Saad Setta H, Elshahat A, Elsherbiny K, Massoud K, Safe I. Platelet-rich plasma versus platelet-poor plasma in the management of chronic diabetic foot ulcers: a comparative study. Int Wound J. 2011;8:307–312. doi: 10.1111/j.1742-481X.2011.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roubelakis MG, Trohatou O, Roubelakis A, Mili E, Kalaitzopoulos I, Papazoglou G, et al. Platelet-rich plasma (PRP) promotes fetal mesenchymal stem/stromal cell migration and wound healing process. Stem Cell Rev. 2014;10:417–428. doi: 10.1007/s12015-013-9494-8. [DOI] [PubMed] [Google Scholar]
- 23.Picard F, Hersant B, Bosc R, Meningaud JP. The growing evidence for the use of platelet-rich plasma on diabetic chronic wounds: a review and a proposal for a new standard care. Wound Repair Regen. 2015;23:638–643. doi: 10.1111/wrr.12317. [DOI] [PubMed] [Google Scholar]
- 24.Villela DL, Santos VL. Evidence on the use of platelet-rich plasma for diabetic ulcer: a systematic review. Growth Factors. 2010;28:111–116. doi: 10.3109/08977190903468185. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Zapata MJ, Martí-Carvajal AJ, Solà I, Expósito JA, Bolíbar I, Rodríguez L, et al. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev. 2016;(5):CD006899. doi: 10.1002/14651858.CD006899.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos-Torrecillas J, García-Martínez O, De Luna-Bertos E, Ocaña-Peinado FM, Ruiz C. Effectiveness of platelet-rich plasma and hyaluronic acid for the treatment and care of pressure ulcers. Biol Res Nurs. 2015;17:152–158. doi: 10.1177/1099800414535840. [DOI] [PubMed] [Google Scholar]
- 27.de Leon JM, Driver VR, Fylling CP, Carter MJ, Anderson C, Wilson J, et al. The clinical relevance of treating chronic wounds with an enhanced near-physiological concentration of platelet-rich plasma gel. Adv Skin Wound Care. 2011;24:357–368. doi: 10.1097/01.ASW.0000403249.85131.6f. [DOI] [PubMed] [Google Scholar]