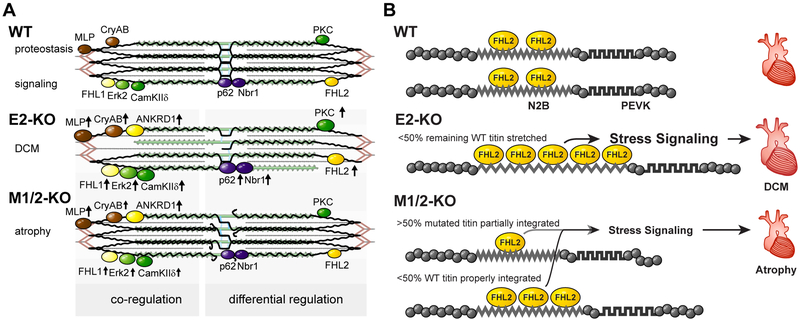
Figure 7. Titin based mechanotransduction.
A) Schematic representation of the sarcomeric and molecular phenotypes of wildtype (WT), E2-KO, and M1/2-KO mice with titin in black, actin in red, and myosin in green. There are fewer titin molecules in the E2 knockout, while truncated titin does not properly integrate into the M1/2 knockout sarcomere. This leads to destabilization of the sarcomere and altered expression of titin binding proteins. Proteins which are co-regulated in both knockouts compared to the wildtype are depicted on the left, differentially regulated proteins on the right. Arrows indicate regulation of expression. B) Model of FHL2 regulation dependent on mechanical strain. Wildtype titin (WT) binds FHL2 and stabilizes the protein. Upon loss of titin in the E2-KO, we suggest that the remaining titin molecules are stretched extensively providing additional FHL2 binding sites to translate strain into a trophic stress signal. E2-KO hearts thus develop dilated cardiomyopathy (DCM). In the M-band KO, the truncated titin protein is less well integrated and should thus experience less strain resulting in reduced binding of FHL2. The remaining titin is stretched less than the remaining titin in E2 knockouts based on the mechanical contribution of truncated titin. The resulting reduction in stress signaling causes atrophy in M1/2-KO hearts.