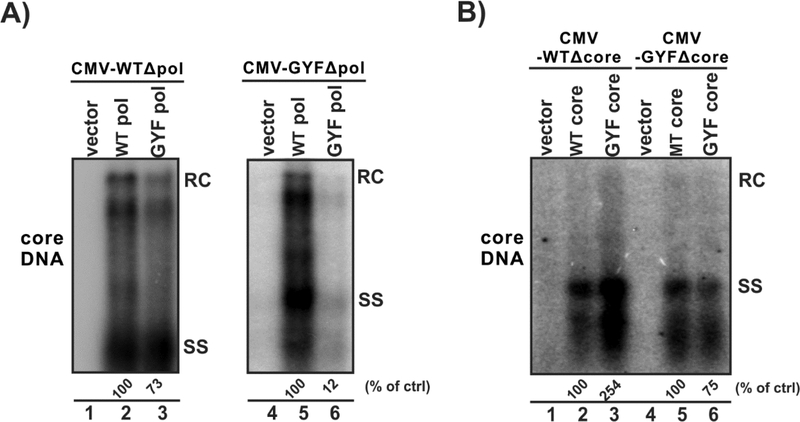
Figure 3. Core protein mutations contribute to the enhanced viral replication of GYF isolate.
(A) HepG2 cells in 12-well-plate were transfected with 0.8 μg of pol-null pCMVHBV-WT (CMV-WTΔpol, lanes 1–3) or 0.8 μg of pol-null pCMVHBV-GYF (CMV-GYFΔpol, lanes 4–6) in combination with 0.8 μg control vector (lanes 1 and 4), or 0.8 μg of plasmid expressing WT pol (lanes 2 and 5), or 0.8 μg of plasmid expressing GYF pol (lanes 3 and 6). (B) HepG2 cells were transfected with 0.8 μg of core-null pCMVHBV-WT (CMV-WTΔcore, lanes 1–3) or 0.8 μg of core-null pCMVHBV-GYF (CMV-GYFΔcore, lanes4–6) in combination with 0.8 μg control vector (lanes 1 and 4), or 0.8 μg of plasmid expressing WT core (lanes 2 and 6), or 0.8 μg of plasmid expressing GYF core (lanes 3 and 5). Cells were harvested at day 5 post-transfection and the cytoplasmic HBV core DNA were detected by Southern blot. The relative levels of core DNA are expressed as the percentage of that in the indicated control (ctrl) samples.