Abstract
Aims and Objectives:
To determine if a weight management intervention (WMI) plus cardiac rehabilitation (CR) compared to CR alone improves outcomes for overweight and obese cardiac revascularization patients.
Background:
Despite participating in cardiac rehabilitation (CR), few cardiac patients lose enough weight to achieve clinically significant cardiovascular disease risk reduction.
Design:
A randomized controlled design was used with measurements at baseline, 4 and 6 months; guided by the CONSORT checklist, see Supplementary File 1. Adults who had undergone either coronary artery bypass surgery (CABS) or percutaneous coronary intervention (PCI) and participated in a rural CR programs were recruited. Subjects were randomized to a 12-week telehealth WMI or control group. The primary outcome was weight loss. Secondary outcomes included physical activity, patient activation, perceived self-efficacy and use of weight management behaviors.
Results:
43 subjects participated, with a mean age of 63 (+/− 9.3) years. The WMI group had significantly more weight loss averaged across the 4 and 6 months of 13.8 (+/− 2.8) pounds compared to the control group [Mean = 7.8 (+/− 2.2) pounds]. There were no significant differences in physical activity (activity counts, or daily minutes in moderate or more intense activity). The WMI group had significantly higher levels of patient activation. They also had significantly higher total scores on the Diet and Exercise Self-Management survey; and subscales that included self-efficacy for specific eating habits and managing diet behavior.
Conclusions:
Findings demonstrated the usefulness and feasibility of using telehealth delivery of the WMI for cardiac rehabilitation participants in rural communities to improve weight management outcomes.
Relevance to Practice:
Study findings underscore the opportunity to further improve weight loss of overweight and obese cardiac participants using a weight management intervention to augment CR participation.
Keywords: Cardiac Rehabilitation, Cardiac Revascularization, Coronary Artery Bypass Surgery, Percutaneous Coronary Intervention, Weight Loss, Weight Management Overweight and Obese Cardiac Patients, rural communities
INTRODUCTION
Cardiac rehabilitation (CR) is the recommended “gold” standard to reduce cardiovascular disease (CVD) secondary risk factors (Hamm et al., 2011; Smith et al., 2011). However, overweight or obese patients often fail to lose enough weight to significantly reduce their CVD risk by CR participation alone (P. A. Ades, Savage, & Harvey-Berino, 2010; Artinian et al., 2010; Gallagher et al., 2012). This is a critical problem since overweight and obese patients comprise 80% and 40% of CR participants respectively (P. A. Ades et al., 2010). Few CR participants have significant weight changes, most have only small weight changes (Gomadam et al., 2016; Manzoni et al., 2011; McKee, Kerins, Fitzgerald, Spain, & Morrison, 2013) and some gain weight during CR (Gomadam et al., 2016). Rural dwelling cardiac patients are at further risk, given the higher propensity of obesity of rural residents (Befort, Nazir, & Perri, 2012; Trivedi, Liu, Probst, & Martin, 2013).
This study was conducted to determine if a telehealth weight management intervention (WMI) plus CR compared to CR alone improves outcomes for overweight and obese cardiac revascularization patients [coronary artery bypass surgery (CABS) and percutaneous coronary intervention (PCI)] in rural communities.
BACKGROUND
National evidence-based guidelines (Hamm et al., 2011; Smith et al., 2011), from the American Heart Association, American College of Cardiology and Association of Cardiovascular and Pulmonary Rehabilitation recommend weight loss counseling and education for overweight or obese cardiac patients as a part of comprehensive CR. A weight loss of 5–10% weight loss is necessary to achieve clinically significant CVD risk reduction (P. A. Ades et al., 2010; Manzoni et al., 2011; Wing et al., 2011). However, typically weight loss interventions are not included in CR (P. A. Ades et al., 2010; P. A. Ades & Savage, 2017). Although CR participants receive standard nutritional consultations, they are not specific to behavioral weight loss intervention. Typically, CR participants only lose ≤ 2% of their body weight (P. A. Ades & Savage, 2017). These small weight losses are not usually sustained after CR (Magalhaes et al., 2013).
Few studies have examined weight loss interventions used in conjunction with standard CR. Overweight participants (n=27) in a nurse-led intervention focused on daily caloric goals, exercise and behavioral modification had significantly (<.001) greater weight loss than CR only participants (n=55); mean weight loss was 4.3 ± 2.8 kg compared to 1.7±2.6 kg (P. D. Savage, Lee, Harvey-Berino, Brochu, & Ades, 2002). Overweight CR participants (n=49) who participated in behavioral weight loss sessions, lost significantly (p<.001) more weight (8.1 ±4.4 vs. 3.6± 4.4 kg) (P. D. Savage, Lakoski, & Ades, 2013). Similarly, in a 12-week behavioral weight loss program following CR completion (Aggarwal et al., 2012), overweight and obese participants (N=44) had significant weight losses.
Other researchers focused on use of intense exercise to promote weight loss. When CR participants engaged in a 4-week intensive CR that included exercise 3 hours per day for 5.5 days/week, they had statistically significant weight losses of 3% (Duarte Freitas et al., 2011). Overweight cardiac patients (N=74) in a high caloric expenditure exercise program had twice the weight loss compared to the CR only group (P. A. Ades et al., 2009).
Only a limited number of weight management interventions for CR participants have been delivered by telehealth. Participants in a CVD risk reduction program (INTERxVent®), delivered by either telephone- or internet, had significantly more weight loss than the CR only group (average of 4.5±8.4 vs 2.3±5.1 pounds) (Gordon et al., 2001). In a web-based program for CAD patients, that included CR participants, participants had a significantly (p<.003) greater weight loss (average of 3.7 vs. 0.47 pounds) (Southard, Southard, & Nuckolls, 2003). Overweight cardiac patients in CR randomized to a pedometer-based telephone coaching program had greater weight loss than the physical activity only group (Sangster et al., 2015). Although these studies demonstrated effectiveness, weight losses achieved by participants were not sustained and/or did not reach weight loss of clinical significance.
This study addresses a critical gap between current cardiac rehabilitation programs and evidence-based recommendations to promote weight management as a secondary CVD risk factor. The purpose of this study was to test a weight management intervention (WMI) to augment weight outcomes of overweight and obese CR participants. Specific aims of this study were to:
Determine whether overweight and obese CR participants in the telehealth WMI group differed from a control group (CR only) on weight outcomes (weight loss and physical activity behaviors) over time at 4 and 6 months after cardiac revascularization.
Examine the differences in weight management perceptions (patient activation, self-efficacy and use of weight loss strategies) of overweight and obese CR participants in the WMI and control groups at 4 months after cardiac revascularization.
Examine feasibility of implementing a WMI for CR participants (treatment delivery, treatment receipt and treatment enactment).
METHODS
Design
This clinical trial used a repeated measures experimental design to examine the impact of a 12-week telehealth WMI on weight loss outcomes (weight loss and physical activity) and weight management perceptions (patient activation, self-efficacy and weight management behaviors) were examined. This study was guided by the CONSORT checklist (Schulz, Altman, & Moher, 2010), see Supplementary File 1. This study specifically targeted cardiac patients residing in rural settings given their higher CVD risk and propensity of obesity (Befort et al., 2012; Trivedi et al., 2013). Both the control and WMI groups participated in a CR program in a rural community following PCI or CABS at a tertiary medical center. All CR programs, including CR programs in this study, provide care based on national standards set forth by the American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) (R. J. Thomas et al., 2010; R. J. Thomas et al., 2018). The 12-week WMI was initiated within 2-weeks after cardiac revascularization. Outcomes for weight loss and physical activity were measured at 4-months and 6-months after cardiac revascularization. At 4-months after cardiac revascularization, perceptions of patient activation, self-efficacy for diet modification and the activity, and diet modification behaviors were measured.
The sample size for this study was based on recommended pilot study sampling (Hertzog, 2008). Estimates with a sample of 25 per group would provide precision of ±.03 to ±.07 at a 68% level of confidence. Forty-three participants completed this study, 22 in the WMI group and 21 in the control group; a final sample well within the 20–40 range considered to be adequate (Kieser & Wassmer, 1996).
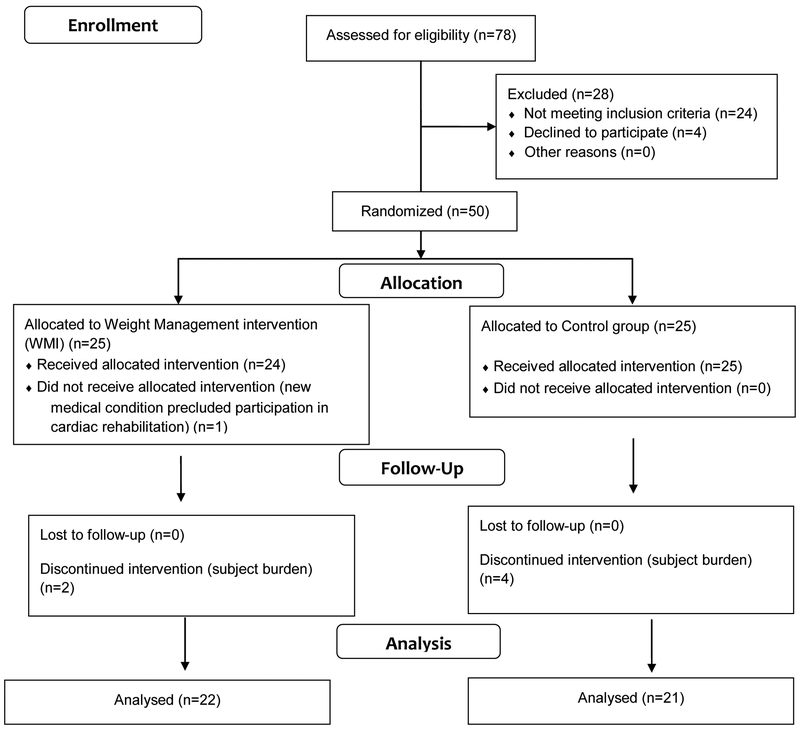
Subjects were recruited from two Midwestern tertiary hospitals. Overweight (BMI > 25 to < 30 kg/m2) or obese (BMI > 30 kg/m2 and <50 kg/m2) CABS or PCI subjects were screened by the research nurse; only four potential subjects declined participation in the study. Refer to Figure 1 for recruitment and retention. Additional inclusion criteria included the ability to speak and read English, available land-line telephone service at home to accommodate use of the Viterion® telehealth device to deliver the telehealth WMI, no cognitive impairment (score of ≤ 8 on the Short-Portable Mental Status Questionnaire) and referral to a CR program in a rural community.
Figure 1.
Subject Recruitment and Randomization
Weight Outcome Measures
Weight loss was measured by BMI and weight change. CR nurses used standardized protocols to obtain weight each data collection time. Weight measures were obtained by CR nurse on calibrated scale at subjects’ CR site. Standardized waist measurements obtained based on placement of tape measure using anatomical placement.
Physical activity behavior was measured by using a triaxial accelerometer, the Actigraph® (model GT3X), with documented reliability and validity for activity measurement (Matthews, 2005). Average daily activity counts based on vector magnitude from all three axes and average daily minutes spent in moderate or higher intensity activity were analyzed. The latter was estimated using the cutpoints recommended by Freedson, Melanson, & Sirard (Freedson, Melanson, & Sirard, 1998). Subjects were instructed on device use related to physical activity and asked to wear the device 24 hours a day for a 7-day time period at 4 and 6 months after PCI or CABS. Data were analyzed only if a participant wore the device for a minimum of 3 days, 10 hours per day during a given collection period.
Managing Weight Loss
Subjects’ perceptions of managing weight loss were collected at 4 months after PCI or CABS when subjects had completed both outpatient CR and the WMI. Perceptions of self-efficacy and patient activation were important to examine usefulness of the underlying mechanisms of the WMI (Bandura, 2004; Bandura, 2005).
Patient activation was measured using the patient activation measure (PAM), with 13-items measuring knowledge, skill and confidence for self-management of health or chronic conditions (Hibbard, Stockard, Mahoney, & Tusler, 2004; Hibbard, Mahoney, Stockard, & Tusler, 2005). The PAM has been used in a variety of cardiac and other populations and has reported reliability and validity (Hibbard et al., 2005; Shively et al., 2005). Cronbach’s alpha for the PAM score in this study was .88.
Self-efficacy for diet behavior modification was measured with the Heart Healthy Eating Self-Efficacy (HHESE) scale, with 43 items using a 1- to 6-point scale; higher scores indicate higher perceived confidence in diet modification. HHESE subscales included self-efficacy for heart-healthy eating habits, situations that may influence eating behaviors and positive outcomes that may result from heart healthy eating (Gaughan, 2003). The HHESE has satisfactory internal consistency and test-retest reliability (Gaughan, 2003). Cronbach’s alphas for the HHESE subscales in this study were .96, .87 and .82 respectively.
Self-Efficacy for exercise was measured using the Cardiac Exercise Self-Efficacy instrument (CESEI), with 16 items using a 5-point response scale measuring perceived exercise behavior confidence. Higher scores indicate greater self-efficacy. The tool has documented internal consistency and reliability; with established predictive and factorial validity (Hickey, Owen, & Froman, 1992). The CESI total score Cronbach’s alpha was .85 in this study.
Use of weight management strategies by participants was measured using the Diet and Exercise Self-Management tool, with documented validity and reliability (Nothwehr & Peterson, 2005; Nothwehr, Dennis, & Haotong Wu, 2007). A 1 to 4--response scale is used for each item, with higher scores indicating use of an increased number of weight management strategies for: diet self-monitoring, food planning, food preparation, portion control, diet management in social interactions, cognitive strategies for diet behaviors, activity self-monitoring, social interaction activity and cognitive strategies for activity behaviors. Cronbach’s alphas for the total scale in this study was .94; and the nine subscales ranged from .46 −.89.
Data Collection
After institutional review board approval, hospital staff screened cardiac revascularization patients for inclusion criteria. All subjects were enrolled prior to hospital discharge by the research nurse and gave written informed consent prior to randomization to WMI or control groups. Demographic and clinical questionnaires were completed. Within a 2-week period prior to start of study, all subjects wore an Actigraph® for 7days between hospital discharge and start of CR. All subjects had their baseline and subsequent 4- and 6-month weight obtained by the CR nurse using a standardized measurement protocol. At 4 and 6 months, subjects wore the Actigraph® for a 7-day period. At 4 months, subjects completed the PAM, HHESE, CESEI, and the Diet and Exercise Self-Management tools.
Subjects received the usual care for post-cardiac revascularization that included participation in a comprehensive CR program. Overweight and obese CR participants received usual education and counseling to manage elevated BMI as a secondary CAD risk factor.
Subjects in the treatment group received the WMI plus CR. The WMI was based on Social Cognitive Theory (SCT)(Bandura, 2004) concepts that posit behavioral change is supported if patients perceived self-efficacy for behavioral change is high. Self-efficacy enhancing interventions for weight management are related to improved modification and maintenance of diet behavior changes (Burke, Dunbar-Jacob, Sereika, & Ewart, 2003). The WMI focused on self-care self-efficacy (judgment regarding capabilities to implement action for weight management) and self-care consequences (benefits and barriers) for weight loss.
WMI Education component.
The WMI was implemented within 2 weeks after PCI or CABS. The research nurse provided the subject with a WMI packet at time of enrollment. The WMI education component included written target caloric goals [recommended total kcal/day for weight loss] and diet portion guidelines. Total daily caloric goals (recommended total kcal/day or caloric reduction for weight loss of each subject) was determined by estimating the daily calories needed to maintain the subject’s baseline weight and subtracting 500–1000 calories/day to achieve a 1 to 2-pound weight loss per week. The WMI also received a handbook for use of portion control guidelines. When the WMI was initiated, the research nurse had a scheduled telephone meeting to review the protocol and review the specifics of the subject’s target caloric goal and use of portion control strategy.
WMI Skill component.
This included a 6-week telehealth component delivered by the Viterion® device, that included 6 modules with 36 telehealth sessions (daily sessions for 3 weeks and 5 sessions per week for the remaining 3 weeks) modeled after the Diabetes Prevention Program (DDP) (DPP Res Grp, 2002). The DDP has previously been successfully adapted for weight loss management (Ma et al., 2013).
WMI Telephone Coaching component.
Telephone coaching sessions were delivered by the research nurse at week 9 and 12. Coaching sessions corresponded with completion of CR, or when subjects completed the majority of the outpatient CR sessions. Coaching sessions(Barrone, Maddux, & Synder, 1997) focused on self-monitoring, self-evaluation and self-regulation for weight reduction behaviors.
Analysis
Repeated measures analysis of covariance (RM-ANCOVA) was used to examine weight management outcomes for weight loss with the corresponding baseline measurement used as a covariate. Physical activity outcomes at 4 and 6 months were analyzed using repeated measures analysis of variance (RM-ANOVA). In addition, to evaluate the components of the intervention, group means in perception of managing weight loss (patient activation, self-efficacy for exercise and diet, and use of weight loss strategies) at 4 months after PCI or CABS were compared using one-way ANOVAs. In all analyses, effect sizes were estimated using η2 (ANOVA) or partial η2 (RM-ANOVA or RM-ANCOVA). Values of .01, .06, and .14 were interpreted as small, medium, or large effects (Cohen, 1988).
Descriptive statistics and open-ended subject responses were summarized to examine the WMI feasibility for treatment delivery, treatment receipt and treatment enactment.
As this study was designed to be a pilot study, we did not expect statistical tests to be significant at conventional alpha levels. We will report p-values and highlight those which are significant at α=.05, but patterns of means and effect sizes also will be discussed.
RESULTS
Participants in the study (N=43) ranged in age from 47–81 years, with a mean age of 63 ± 9.3 years. There were 30 males (70%) and 13 females (30%); 33 had undergone a PCI procedure and 10 had CABS. Most subjects were married (86%, n=37) and had an average of 12.9 ± 1.8 years of formal education; 56% (n=24) of the subjects worked outside of the home and 35% were retired. Subjects’ mean body mass index (BMI) was 35.1 (± 5.5), ranging from 26.1–54.7. Subjects participated in CR programs in rural communities. All subjects completed outpatient CR; they attended an average of 22 (± 3.4) phase 2 CR sessions. There were no significant differences in baseline demographic or clinical characteristics by group, using chi-square and Fisher’s exact test, refer to Table 1. Data collection occurred over an 18-month period to accommodate study follow-up periods (4- and 6-months) as well as feasibility follow-up to subject at 9- and 12-months after cardiac revascularization.
Table 1.
Demographic and Clinical Characteristics of Study Sample (N = 43)
| Gender | ||||
| Male | 30 (70) | 17 (77) | 13 (62) | |
| Female | 13 (30) | 5 (23) | 8 (38) | χ2 = .273 |
| Marital Status | ||||
| Married | 37 (86) | 20 (91) | 17 (81) | |
| Not Married (single, widowed, or divorced) | 6 (14) | 2 (9) | 4 (19) | Fisher Exact test p = .412 |
| Employment Status | ||||
| Currently working | 24 (56) | 14 (64) | 10 (48) | |
| Not currently working or retired | 19 (44) | 8 (36) | 11 (52) | χ2 =.290 |
| Cardiac Revascularization | ||||
| PCI | 33 (77) | 16 (73) | 17 (81) | |
| CABS | 10 (23) | 6 (27) | 4 (19) | χ2 =.721 |
Weight Management Outcomes
Descriptive statistics by group and results of statistical tests of group differences are presented, see Table 2 and 3. For analyses of weight loss outcomes, the only effect significant at the .05 level was the group comparison of weight change from baseline. For the WMI group, the weight loss averaged across 4 and 6 months (the marginal group mean, adjusted for baseline weight) was 13.7 pounds. This was significantly greater than the 8 pounds average weight loss of the control group. This weight change differences represents a medium-to-large effect (partial η2=.09). Expressed as percentage of baseline weight, the WMI group had an average loss of 5.9%, while the control group, on average, lost 3.6% of their baseline weight. Although the pattern of means favored the WMI group on BMI, group effects on these outcomes were small (partial η2 of .02 - .05) and non-significant.
Table 2.
Mean Scores for Weight Management Outcomes
| Group | Sample n | Means (SD) Baseline | Means (SD) 4-month | Means (SD) 6-Months |
|---|---|---|---|---|
| Body Mass Index (BMI) (Mean BMI) | ||||
| WMI | 20 | 37.6 (6.0) | 35.8 (6.0) | 35.2 (6.0) |
| Control | 21 | 32.8 (4.0) | 31.8 (3.9) | 31.3 (4.1) |
| Weight Change (# of Pounds) | ||||
| WMI | 20 | 242.8 (38.0)a | −11.9 (7.0) | −15.8 (9.7) |
| Control | 21 | 217.7 (35.9)a | −6.2 (7.9) | −9.3 (11.4) |
| Physical Activity (Actigraph®): Vector Magnitude (Mean # counts/day) | ||||
| WMI | 6 | ----- | 432703 (206537) | 513696 (217882) |
| Control | 10 | ----- | 395739 (96960) | 387911 (164664) |
| Physical Activity (Actigraph®): Moderate or More Intense Level of Activity (Mean # minutes/day) | ||||
| WMI | 6 | ----- | 14.4 (16.1) | 24.0 (34.1) |
| Control | 10 | ----- | 11.0 (7.9) | 14.2 (19.4) |
Baseline weight in pounds was used as a covariate in analysis of weight change outcome.
Table 3.
Weight Management Outcomes over Time
| ANCOVA with Repeated Measures for Weight Management Outcomes: BMI, Waist Measurement, Weight Change, and Diet Behavior with Baseline Measures as Covariates | |||||||||
| Variable | Group | Time | Group* Time | ||||||
| F (df) | p | ηp2 | F (df) | p | ηp2 | F (df) | p | ηp2 | |
| BMI | 1.9 (1,38) | .18 | .05 | .06 (1,38) | .81 | <.01 | .00 (1,38) | .99 | <.01 |
| Weight Change from baseline to 4 months and baseline to 6 months | 4.0 (1,38) | .05* | .09 | .45 (1,38) | .51 | .01 | .19 (1,38) | .67 | <.01 |
| ANOVA with Repeated Measures for Weight Management Outcomes: Physical Activity | |||||||||
| Variable | Group | Time | Group* Time | ||||||
| F (df) | p | ηp2 | F (df) | p | ηp2 | F (df) | p | ηp2 | |
| Physical Activity Behavior (Actigraph®): Mean Vector Magnitude Counts | 1.1 (1,14) | .32 | .07 | 1.2 (1,14) | .29 | .08 | 1.8 (1,14) | .21 | .11 |
| Physical Activity Behavior (Actigraph®): Mean Levels of Moderate Activity | .56 (1,14) | .47 | .04 | 1.5 (1,14) | .25 | .10 | .37 (1,14) | .56 | .03 |
Note: ηp2 = partial eta squared
p≤.05
All participants were asked to wear the Actigraph® for 24 hours per day for 7 days at month 4 and month 6. Of the 16 participants with data at both times, the number of days worn at each time ranged from 3–6 (mean=3.7) in the WMI group (n=22) and from 3–7 (mean=4.8) in the control group (n=21). There were no significant effects in the RM-ANOVA on either activity counts (vector magnitude) or daily minutes of moderate or more intense activity. Descriptively, the WMI group was more active at both 4 and 6 months. At 6 months, the WMI group, on average, spent almost twice the average minutes per day in moderate or more intense levels of activity compared to the control group (24.0 minutes compared to 14.2 minutes, respectively). Group effects were in the moderate range (η2 = .04 - .07).
Weight Management Perceptions
Descriptive statistics and results of the ANOVAs for comparing the WMI and control groups at 4 months on the tools measuring perceptions of managing weight loss management are summarized on Table 4. Perceptions of subjects’ patient activation for managing their own healthcare, as measured by the PAM at 4 months, demonstrated a significant group difference (F(1,38)=5.3, p=.02). The WMI group had higher activation scores than control subjects (64.7 ± 9.0 vs. 58.4 ± 8.3); and a medium-to-large effect size of η2=.12.
Table 4.
Perceptions of Managing Weight Loss: Descriptive and ANOVA Analyses for PAM, HHESE scale, CESEI, and Diet and Exercise Self-Management Survey at 4-months after Cardiac Revascularization
| Patient Activation Measure (PAM) | |||||||
| n |
WMI Group M (SD) Control M (SD) |
F (df) | p | η2 | |||
| PAM | 19 21 |
64.7 (9.0) 58.4 (8.3) |
5.3 (1,38) | .027* | .12 | ||
| Heart Healthy Eating Self-Efficacy Scale (HHESE) | |||||||
| Self-efficacy beliefs for specific eating habits | 19 19 |
5.3 (.26) 4.7 (.71) |
12.6 (1,36) | .001* | .26 | ||
| Self-Efficacy for Situational/Environmental diet management behaviors | 19 19 |
5.2 (.27) 4.9 (.37) |
8.6 (1,36) | .006* | .19 | ||
| Self-efficacy for diet management outcome expectations | 19 19 |
4.9 (.48) 4.7 (.45) |
2.1 (1,36) | .15 | .06 | ||
| Cardiac Exercise Self-Efficacy Index (CESEI) | |||||||
| Total Mean Score | 19 20 |
4.6 (.38) 4.4 (.29) |
2.4 (1,38) | .13 | .06 | ||
| Diet and Exercise Self-Management Survey | |||||||
| Total Mean Score | 21 19 |
2.4 (.32) 2.1 (.28) |
10.7 (1,38) | .002* | .22 | ||
| Monitoring strategies: diet | 21 19 |
2.6 (.62) 1.9 (.51) |
11.3 (1,38) | .002* | .23 | ||
| Planning strategies | 21 19 |
2.4 (.37) 2.0 (.41) |
9.7 (1,38) | .003* | .20 | ||
| Preparation/buying food strategies | 21 19 |
3.8 (.32) 3.3 (.45) |
12.2 (1,38) | .001* | .24 | ||
| Portion control strategies | 21 19 |
3.0 (.38) 2.7 (.36) |
7.8 (1,38) | .008* | .17 | ||
| Social interaction strategies: diet | 21 19 |
2.4 (.62) 2.2 (.46) |
1.1 (1,38) | .30 | .03 | ||
| Cognitive strategies: diet | 21 19 |
1.9 (.45) 1.7 (.46) |
2.5 (1,38) | .12 | .06 | ||
| Monitoring strategies: activity | 21 19 |
2.2 (.42) 2.0 (.33) |
4.5 (1,38) | .04* | .11 | ||
| Social interaction strategies: activity | 21 19 |
1.8 (.43) 1.6 (.33) |
2.4 (1,38) | .13 | .06 | ||
| Cognitive strategies: activity | 21 19 |
1.9 (.38) 1.7 (.37) |
3.2 (1,38) | .08 | .08 | ||
Note: η2 = eta squared
p≤.05
There were also significant differences between the groups on two of three self-efficacy health heart eating subscales. Participants in WMI had significantly higher perceived self-efficacy for specific eating habits [F (1,36)=12.6, p=.001] and managing diet behavior in different situations [F (1,36)=8.6, p=.006]; both had large effect sizes (η2 ranging from .19 −.26).
The CESI measured self-efficacy for exercise, or the perceptions of confidence to engage in exercise behaviors as recommended for cardiac patient. There was no significant differences between groups, although the estimated effect size was in the moderate range (η2=.06).
Use of weight management strategies demonstrated the WMI group had a statistically significantly higher overall total mean score [F(1,38)=10.7, p=.002], with a large effect size (η2 =.22). Five weight management strategy use subscales of monitoring diet, planning diet, preparing and buying food and portion control were significantly higher among WMI group. Analysis of the remaining four subscales were not significantly different by group, although moderate effect sizes were estimated for group differences in cognitive strategies used related to diet (η2=.06) and cognitive (η2=.08) and social interaction strategies related to activity (η2=.06).
Feasibility
All WMI participants (100%) received one-to-one education on the target caloric goals [recommended total kcal/day for weight loss for each individual participant] and written copy of diet portion guidelines. Treatment delivered was measured by the number of intervention sessions delivered by the Viterion® and treatment receipt was the number of WMI sessions accessed by the participants. Four of the 22 WMI subjects (18%) completed < 5 sessions (e.g., less than one week) of the telehealth component. Of the remaining subjects, 8 subjects (36%) completed all 36 sessions; and 10 of (46%) completed from 30–36 sessions. Telephone coaching for the WMI subjects was delivered, with the 2 telephone coaching sessions delivered during week 9 and week 12 of the intervention. All subjects (100%) received and participated in the coaching sessions of the intervention, average telephone coaching sessions were from 10–15 minutes.
Treatment enactment or the reported perception of usefulness of the strategies and participant satisfaction with intervention (satisfaction with the mode of delivery, satisfaction with content of intervention, satisfaction with helpfulness of sessions—both telehealth sessions and virtual coaching) was evaluated at 9- and 12-months after their cardiac event. At 9 months after their cardiac event, 11 subjects in the WMI group were able to be contacted by telephone and provided a self-report of their weight. Of these subjects, comparing their reported weight to their 6-month weight, six had further weight loss; while four had maintained their weight and one had gained weight. In the control group, 10 subjects were contacted and reported their weight. In comparison to their 6-month weight, two subjects had lost weight; while five subjects weighed the same and three subjects gained weight.
At 12 months after their cardiac event, 21 subjects in the WMI group were able to be contacted and provided a self-report of their weight. Comparing their reported weight to their 6-month weight, 10 had further weight loss; while nine had maintained their weight and two had gained weight. In the control group, 13 subjects were contacted and provided their weight. Subjects’ reported weights were compared to their 6-month weight. Three had lost weight; while eight subjects weighed the same and two had gained weight.
Subjects in the WMI group were queried about their satisfaction at 9-months; WMI subjects (n=11) had a mean satisfaction score of 6.19 ±1.8 on a 0–10 scale (a higher score indicates higher satisfaction) for the weight management intervention. At 12-months, WMI participants (n=21) had a mean satisfaction score of 6.3 ±1.9.
Participants were also asked about their perceptions regarding the length of the WMI. At 9-months (n=11), 4 subjects indicated the intervention was “too long”, 1 subject thought it was “too short” and 6 subjects thought it was “just about right” in length. At 12-months (n=21), 14 subjects thought the intervention was “too long”, one subject reported the intervention to be “too short” and six subjects reported their perception as length of intervention being “just right”. During the 9- and 12-month follow-up session of subjects in the pilot study, open ended questions were used to elicit feedback on perceived strategies being used to manage weight. The feedback indicated that the most frequently cited strategies were: use of portion control with dietary intake, mindfulness of what one is eating, making diet intake focus a priority daily, engaging in activity that is more physical and/or exercise.
DISCUSSION
Findings from this study further inform cardiac rehabilitation research examining interventions to augment cardiac patients’ weight loss outcomes as recommended by experts (P. A. Ades et al., 2010). This concept is not unlike providing a formal program for smoking cessation (p.2679) as a core component of CR programs(Balady et al., 2007). Delivering a WMI in conjunction with CR for cardiac patients in this study was found to be both feasible to deliver and implement; and useful for the rural WMI participants. Use of the telehealth WMI standardized the intervention implementation and did not rely on delivery of the WMI by the CR staff. Nor did the intervention require additional travel to the CR program site to participate in this WMI program.
The increased weight loss by the WMI group is consistent with other studies that reported greater weight loss when interventions were implemented in conjunction with CR (P. A. Ades et al., 2009; Duarte Freitas et al., 2011; Jiang, Sit, & Wong, 2007; Magalhaes et al., 2013). However, like other WMI studies, the weight losses were modest. Furthermore, sustained weight loss associated with the WMI used in this study is unknown given the limited follow-up of subjects in this pilot study.
Subjects in the study had significantly higher patient activation, self-efficacy for diet behaviors and use of weight management strategies by the WMI group, with large effect sizes. These findings support the importance of patient engagement to activate behavior change(Dietz et al., 2015) and improve self-efficacy for self-management that may be useful in future studies to modify the intervention for greater effect size.
Study limitations constrain the generalizability to the larger population of overweight and obese patients in CR, as the sample size was small (N=43), with subjects recruited from two Midwestern, tertiary hospitals. In this pilot study, the perceptions of managing weight loss (patient activation, self-efficacy for diet and physical activity and uses of cognitive behavioral strategies for weight management) were measured only one-time coinciding with completion of the telehealth intervention. Furthermore, the study sample was not ethnically or economically diverse. Physical activity, measured by the Actigraph®, and the measurement of weight were the only objectively measured outcome variables; all other measures were based on participants’ self-report.
CONCLUSIONS
In summary, the implementation of a telehealth WMI intervention in conjunction with CR provided a simple, effective approach to augment weight loss. This study demonstrated the feasibility of implementing a specific WMI program component for CR participants that could be implemented in conjunction with standard CR in response to the national guidelines to use behavioral interventions to promote adherence and self-management skills in weight management (P. A. Ades et al., 2010; Hamm et al., 2011). Furthermore, the use of telehealth delivery of the WMI provides access to the intervention by CR participants regardless of location in urban or rural communities. Further study is warranted, using a fully powered study to evaluate the efficacy of using a WMI integrated in CR programs. Efficacious weight management programs should be disseminated and implemented into CR as a standard approach to improve weight loss outcomes for overweight and obese cardiac patients.
RELEVANCE TO CLINICAL PRACTICE
Given the paucity of research evidence on managing weight as a modifiable cardiac risk factor for CR participants, this study demonstrates the feasibility of integrating a WMI for overweight and obese cardiac patients participating in CR. Use of telehealth strategies may be useful to deliver weight management interventions for cardiac patients in rural communities.
Supplementary Material
What does this paper contribute to the wider global clinical community?
Overweight and obese cardiac patients often fail to lose enough weight in cardiac rehabilitation to clinically reduce their cardiac risk.
Strategies to support weight loss, beyond standard cardiac rehabilitation components are needed to attain clinically significant weight loss.
The WMI used in this study is an exemplar of types of interventions that may be useful with further study to augment weight loss outcomes of overweight and obese cardiac rehabilitation participants.
Acknowledgements:
Study Supported by NIH/NINR P20 NR011404 Interdisciplinary Healthy Heart Center: Linking Rural Populations by Technology
Contributor Information
Susan Barnason, University of Nebraska Medical Center, College of Nursing-Lincoln Division.
Lani Zimmerman, University of Nebraska Medical Center, College of Nursing-Lincoln Division.
Paula Schulz, University of Nebraska Medical Center, College of Nursing-Lincoln Division.
Carol Pullen, University of Nebraska Medical Center, College of Nursing-Omaha Division.
Sue Schuelke, University of Nebraska Medical Center, College of Nursing-Lincoln Division.
REFERENCES
- Ades PA, Savage PD, Toth MJ, Harvey-Berino J, Schneider DJ, Bunn JY, … Ludlow M (2009). High-calorie-expenditure exercise: A new approach to cardiac rehabilitation for overweight coronary patients. Circulation, 119(20), 2671–2678. doi: 10.1161/CIRCULATIONAHA.108.834184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ades PA, & Savage PD (2017). Obesity in coronary heart disease: An unaddressed behavioral risk factor. Preventive Medicine, doi: 10.1016/j.ypmed.2017.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ades PA, Savage PD, & Harvey-Berino J (2010). The treatment of obesity in cardiac rehabilitation. Journal of Cardiopulmonary Rehabilitation and Prevention, 30(5), 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal S, Arena R, Cuda L, Hauer T, Martin BJ, Austford L, & Stone JA (2012). The independent effect of traditional cardiac rehabilitation and the LEARN program on weight loss: A comparative analysis. Journal of Cardiopulmonary Rehabilitation and Prevention, 32(1), 48–52. doi:10.1097/HCR.0b013e31823f2da1 ; 10.1097/HCR.0b013e31823f2da1 10.1097/HCR.0b013e31823f2da1; 10.1097/HCR.0b013e31823f2da1 [DOI] [PubMed] [Google Scholar]
- Artinian NT, Fletcher GF, Mozaffarian D, Kris-Etherton P, Van Horn L, Lichtenstein AH, … American Heart Association Prevention Committee of the Council on Cardiovascular Nursing. (2010). Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: A scientific statement from the american heart association. Circulation, 122(4), 406–441. doi: 10.1161/CIR.0b013e3181e8edf1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balady GJ, Williams MA, Ades PA, Bittner V, Comoss P, Foody JM, … Southard D (2007). Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: A scientific statement from the american heart association exercise, cardiac rehabilitation, and prevention committee, the council on clinical cardiology; the councils on cardiovascular nursing, epidemiology and prevention, and nutrition, physical activity, and metabolism; and the american association of cardiovascular and pulmonary rehabilitation. Circulation, 115(20), 2675–2682. [DOI] [PubMed] [Google Scholar]
- Bandura A (2004). Health promotion by social cognitive means. Health Education & Behavior, 31(2), 143–164. [DOI] [PubMed] [Google Scholar]
- Bandura A (2005). The primacy of self-regulation in health promotion. Applied Psychology, 54(2), 245–254. [Google Scholar]
- Barrone DF, Maddux JE, & Synder CR (1997). Social cognitive psychology: History and current domains. New York: Plenam, [Google Scholar]
- Befort CA, Nazir N, & Perri MG (2012). Prevalence of obesity among adults from rural and urban areas of the united states: Findings from NHANES (2005–2008). The Journal of Rural Health: Official Journal of the American Rural Health Association and the National Rural Health Care Association, 28(4), 392–397. doi: 10.1111/j.1748-0361.2012.00411.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke LE, Dunbar-Jacob J, Sereika S, & Ewart CK (2003). Development and testing of the cholesterol-lowering diet self-efficacy scale. European Journal of Cardiovascular Nursing, 2(4), 265–273. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). In Cohen J (Ed.), Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, New Jersey: Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Dietz WH, Baur LA, Hall K, Puhl RM, Taveras EM, Uauy R, & Kopelman P (2015). Management of obesity: Improvement of health-care training and systems for prevention and care. Lancet, doi: 10.1016/S0140-6736(14)61748-7 [DOI] [PubMed] [Google Scholar]
- DPP Res Grp. (2002). The diabetes prevention program (DPP) - description of lifestyle intervention. Diabetes Care, 25(12), 2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte Freitas P, Haida A, Bousquet M, Richard L, Mauriege P, & Guiraud T (2011). Short-term impact of a 4-week intensive cardiac rehabilitation program on quality of life and anxiety-depression. Annals of Physical and Rehabilitation Medicine, 54(3), 132–143. doi:10.1016/j.rehab.2011.02.001 ; 10.1016/j.rehab.2011.02.001 10.1016/j.rehab.2011.02.001; 10.1016/j.rehab.2011.02.001 [DOI] [PubMed] [Google Scholar]
- Freedson PS, Melanson E, & Sirard J (1998). Calibration of the computer science and applications, inc. accelerometer. Medicine & Science in Sports & Exercise., 30(5), 777–781. [DOI] [PubMed] [Google Scholar]
- Gallagher R, Kirkness A, Zelestis E, Hollams D, Kneale C, Armari E, … Tofler G (2012). A randomised trial of a weight loss intervention for overweight and obese people diagnosed with coronary heart disease and/or type 2 diabetes. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine, 44(1), 119–128. doi: 10.1007/s12160-012-9369-2 [DOI] [PubMed] [Google Scholar]
- Gaughan ME (2003). Heart healthy eating self-efficacy. Topics in Clinical Nutrition, 18(4; 4), 229–244. [Google Scholar]
- Gomadam PS, Douglas CJ, Sacrinty MT, Brady MM, Paladenech CC, & Robinson KC (2016). Degree and direction of change of body weight in cardiac rehabilitation and impact on exercise capacity and cardiac risk factors. American Journal of Cardiology, 117(4), 580–584. doi: 10.1016/j.amjcard.2015.11.045 [DOI] [PubMed] [Google Scholar]
- Gordon NF, Salmon RD, Mitchell BS, Faircloth GC, Levinrad LI, Salmon S, … Reid KS (2001). Innovative approaches to comprehensive cardiovascular disease risk reduction in clinical and community-based settings. Current Atherosclerosis Reports, 3(6), 498–506. [DOI] [PubMed] [Google Scholar]
- Hamm LF, Sanderson BK, Ades PA, Berra K, Kaminsky LA, Roitman JL, & Williams MA (2011). Core competencies for cardiac rehabilitation/secondary prevention professionals: 2010 update: Position statement of the american association of cardiovascular and pulmonary rehabilitation. Journal of Cardiopulmonary Rehabilitation and Prevention, 31(1), 2–10. doi: 10.1097/HCR.0b013e318203999d [DOI] [PubMed] [Google Scholar]
- Hertzog MA (2008). Considerations in determining sample size for pilot studies. Research in Nursing & Health, 31(2), 180–191. doi: 10.1002/nur.20247 [DOI] [PubMed] [Google Scholar]
- Hibbard JH, Mahoney ER, Stockard J, & Tusler M (2005). Development and testing of a short form of the patient activation measure. Health Services Research, 40(6), 1918–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbard JH, Stockard J, Mahoney ER, & Tusler M (2004). Development of the patient activation measure (PAM): Conceptualizing and measuring activation in patients and consumers. Health Services Research, 39(4), 1005–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey ML, Owen SV, & Froman RD (1992). Instrument development: Cardiac diet and exercise self-efficacy. Nursing Research, 41(6), 347–351. [PubMed] [Google Scholar]
- Jiang X, Sit JW, & Wong TK (2007). A nurse-led cardiac rehabilitation programme improves health behaviours and cardiac physiological risk parameters: Evidence from chengdu, china. Journal of Clinical Nursing, 16(10), 1886–1897. doi: 10.1111/j.1365-2702.2007.01838.x [DOI] [PubMed] [Google Scholar]
- Kieser M, & Wassmer G (1996). On the use of the upper confidence limit for the variance from a pilot sample for sample size determination. Biometrical Journal, 38(8), 941–949. [Google Scholar]
- Ma J, Yank V, Xiao L, Lavori PW, Wilson SR, Rosas LG, & Stafford RS (2013). Translating the diabetes prevention program lifestyle intervention for weight loss into primary care: A randomized trial. JAMA Internal Medicine, 173(2), 113–121. doi:10.1001/2013.jamainternmed.987 ; 10.1001/2013.jamainternmed.987 10.1001/2013.jamainternmed.987; 10.1001/2013.jamainternmed.987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes S, Viamonte S, Miguel Ribeiro M, Barreira A, Fernandes P, Torres S, & Lopes Gomes J (2013). Long-term effects of a cardiac rehabilitation program in the control of cardiovascular risk factors. [Efeitos a longo prazo de um programa de reabilitacao cardiaca no controlo dos fatores de risco cardiovasculares] Revista Portuguesa De Cardiologia : Orgao Oficial Da Sociedade Portuguesa De Cardiologia = Portuguese Journal of Cardiology : An Official Journal of the Portuguese Society of Cardiology, doi:10.1016/j.repc.2012.08.005 ; 10.1016/j.repc.2012.08.005 10.1016/j.repc.2012.08.005; 10.1016/j.repc.2012.08.005 [DOI] [PubMed] [Google Scholar]
- Manzoni GM, Villa V, Compare A, Castelnuovo G, Nibbio F, Titon AM, … Gondoni LA (2011). Short-term effects of a multi-disciplinary cardiac rehabilitation programme on psychological well-being, exercise capacity and weight in a sample of obese in-patients with coronary heart disease: A practice-level study. Psychology, Health & Medicine, 16(2), 178–189. doi:10.1080/13548506.2010.542167 ; 10.1080/13548506.2010.542167 10.1080/13548506.2010.542167; 10.1080/13548506.2010.542167 [DOI] [PubMed] [Google Scholar]
- Matthews CE (2005). Calibration of accelerometer output for adults. Medicine & Science in Sports & Exercise, 37(11), S512–S522. [DOI] [PubMed] [Google Scholar]
- McKee G, Kerins M, Fitzgerald G, Spain M, & Morrison K (2013). Factors that influence obesity, functional capacity, anxiety and depression outcomes following a phase III cardiac rehabilitation programme. Journal of Clinical Nursing, 22(19), 2758–2767. doi: 10.1111/jocn.12233 [DOI] [PubMed] [Google Scholar]
- Nothwehr F, Dennis L, & Haotong Wu. (2007). Measurement of behavioral objectives for weight management. Health Education & Behavior, 34(5), 793–809. [DOI] [PubMed] [Google Scholar]
- Nothwehr F, & Peterson NA (2005). Healthy eating and exercise: Strategies for weight management in the rural midwest. Health Education & Behavior, 32(2), 253. [DOI] [PubMed] [Google Scholar]
- Sangster J, Furber S, Allman-Farinelli M, Phongsavan P, Redfern J, Haas M, … Bauman A (2015). Effectiveness of a pedometer-based telephone coaching program on weight and physical activity for people referred to a cardiac rehabilitation program: A randomized controlled trial. Journal of Cardiopulmonary Rehabilitation and Prevention, 35(2), 124–129. doi: 10.1097/HCR.0000000000000082 [DOI] [PubMed] [Google Scholar]
- Savage PD, Lee M, Harvey-Berino J, Brochu M, & Ades PA (2002). Weight reduction in the cardiac rehabilitation setting. Journal of Cardiopulmonary Rehabilitation, 22(3), 154–160. [DOI] [PubMed] [Google Scholar]
- Savage PD, Lakoski SG, & Ades PA (2013). Course of body weight from hospitalization to exit from cardiac rehabilitation. Journal of Cardiopulmonary Rehabilitation and Prevention, 33(5), 274–280. doi: 10.1097/HCR.0b013e31829b6e9f [DOI] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, & Moher D (2010). CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ: British Medical Journal, 340(7748), c332–c332. doi: 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively M, Kodiath M, Smith TL, Kelly A, Bone P, Fetterly L, … Dracup K (2005). Effect of behavioral management on quality of life in mild heart failure: A randomized controlled trial. Patient Education and Counseling, 58(1), 27–34. doi: 10.1016/j.pec.2004.06.007 [DOI] [PubMed] [Google Scholar]
- Smith SJ, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, … Taubert KA (2011). AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: A guideline from the American Heart Association and American College of Cardiology foundation. Circulation, 124(22), 2458–2473. [DOI] [PubMed] [Google Scholar]
- Southard BH, Southard DR, & Nuckolls J (2003). Clinical trial of an internet-based case management system for secondary prevention of heart disease. Journal of Cardiopulmonary Rehabilitation, 23(5), 341–348. [DOI] [PubMed] [Google Scholar]
- Thomas RJ, King M, Lui K, Oldridge N, Pina IL, Spertus J, & ACCFAHA Task Force on Performance Measures. (2010). AACVPR/ACCF/AHA 2010 update: Performance measures on cardiac rehabilitation for referral to cardiac rehabilitation/secondary prevention services: A report of the American Association of Cardiovascular and Pulmonary Rehabilitation and the American College of Cardiology Foundation/American Heart Association task force on performance measures (writing committee to develop clinical performance measures for cardiac rehabilitation). Journal of Cardiopulmonary Rehabilitation and Prevention, 30(5), 279–288. doi:10.1097/HCR.0b013e3181f5e36f ; 10.1097/HCR.0b013e3181f5e36f 10.1097/HCR.0b013e3181f5e36f; 10.1097/HCR.0b013e3181f5e36f [DOI] [PubMed] [Google Scholar]
- Thomas RJ, Balady G, Banka G, Beckie TM, Chiu J, Gokak S, … Wang TY (2018). 2018 ACC/AHA clinical performance and quality measures for cardiac rehabilitation: A report of the American College of Cardiology/American Heart Association task force on performance measures. Journal of the American College of Cardiology, 71(16), 1814–1837. doi: 10.1016/j.jacc.2018.01.004 [DOI] [PubMed] [Google Scholar]
- Trivedi T, Liu J, Probst JC, & Martin AB (2013). The metabolic syndrome: Are rural residents at increased risk? The Journal of Rural Health, 29(2), 188–197. [DOI] [PubMed] [Google Scholar]
- Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, … Wagenknecht L (2011). Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care, 34(7), 1481–1486. doi: 10.2337/dc10-2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.