Abstract
Background
Proton pump inhibitors (PPIs) have been linked to acute kidney injury (AKI) and chronic kidney disease (CKD); however, current evidence has only been evaluated in a small number of studies with short follow-up periods. This study examined the association between PPI use and risk of incident AKI and CKD in a large, population-based health-maintenance organization (HMO) cohort.
Methods
Patients aged 18 years or older, without evidence of pre-existing renal disease, started on PPI therapy, and continuously enrolled for at least 12 months between July 1993 and September 2008 were identified in an HMO database. Incidences of AKI and CKD were defined using documented International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) codes or a glomerular filtration rate less than 60 ml/min/1.73 m2 after initiation of PPI therapy. Patients with AKI were followed for up to 90 days (cohort 1) and patients with CKD required at least 1 year of follow-up (cohort 2). Multivariable logistic regression analyses were used to adjust for differences in demographics (excluding race), comorbidities, and medication use between groups.
Results
In 93,335 patients in the AKI cohort, 16,593 of whom were exposed to PPIs, the incidence rate of AKI was higher in the PPI group than nonusers (36.4 vs. 3.54 per 1000 person-years, p<0.0001, respectively). In adjusted models, PPI exposure was associated with an increased risk of AKI (Adjusted Odds Ratio (aOR) 4.35; 95% Confidence Interval (CI) 3.14–6.04; p<0.0001). In 84,600 patients in the CKD cohort, 14,514 of whom were exposed to PPIs, the incidence rate of CKD was higher in the PPI group than nonusers (34.3 vs. 8.75 per 1000 person-years, p<0.0001, respectively). In adjusted models, PPIs were associated with a higher risk of CKD compared to controls (aOR 1.20; 95% CI 1.12–1.28; p<0.0001). Associations between PPI use and AKI and CKD persisted in propensity score-matched analyses.
Conclusion
Proton pump inhibitors use is associated with an increased risk of incident AKI and CKD. This relationship could have a considerable public health impact; therefore, health care provider education and deprescribing initiatives will be necessary to raise awareness and reduce health care burden.
Keywords: Acute kidney injury, chronic kidney disease, observational study, proton pump inhibitors
Proton pump inhibitors (PPIs) are widely used to treat acid-related gastrointestinal disorders, with an estimated 113 million prescriptions filled in the United States in 2008, totaling $13.9 billion.1 Although PPIs are one of the most commonly prescribed medications, 25% to 70% of these prescriptions are estimated to have no appropriate indication.2 Indications such as gastroesophageal reflux disease require only short-term treatment with PPIs (i.e., up to 4–8 weeks), but chronic use appears to be common.2–4 About 40% to 55% of primary care patients and up to 65% of hospitalized patients have no documented ongoing indications for PPIs.5–8 Consequently, patients often take these medications without benefit and are therefore subject to unnecessary adverse events.
Proton pump inhibitors are generally considered to be a safe class of drugs; however, inappropriate prescribing can contribute to polypharmacy with its inherent risks of nonadherence, prescribing cascades, adverse reactions, medication errors, drug interactions, emergency department visits, and hospitalizations.9, 10 Further, several observational studies have linked PPIs to adverse health outcomes including hip fractures, enteric infections, acute interstitial nephritis, and community-acquired pneumonia, as well as an increased risk of mortality among users.11–15 A growing concern is that PPI use may be a risk factor for chronic kidney disease (CKD), potentially mediated by recurrent acute kidney injury (AKI).16, 17 The mechanism for this relationship is currently unknown, however, possible mechanisms include development of acute interstitial nephritis, a hypersensitivity reaction that can lead to a decline in glomerular filtration rate and adverse renal outcomes.18 Other possible mechanisms include inhibition of the lysosomal proton pump, with decreased nitric oxide synthesis and increased generation of superoxide anion, or hypomagnesemia, which could lead to increased secretion of inflammatory and atherogenic markers. PPIs have been shown to be associated with adverse kidney outcomes; however, a recent systematic review noted that this evidence was of low or insufficient quality.19–23 Therefore, additional studies evaluating the relationship between PPI use and renal disease are necessary, especially considering the increasing global use of PPIs, as this relationship could pose a substantial disease and financial burden to health care systems.
The objective of this study was to determine the association between PPI use and incident AKI and CKD in a general population. A local health-maintenance organization (HMO) database was used to build two cohorts of new PPI users and additional cohorts for sensitivity analyses, including 1:1 propensity score-matched cohorts with predictor variables of age, gender, comorbidities, and concomitant medication use. In this way, the association between PPI exposure and risk of incident AKI and CKD was examined among persons without kidney disease at baseline.
Methods
Claims data from a local HMO in Western New York was used to examine the relationships between acute and chronic kidney disease and prescription PPI use. The database covered a cohort of patients from July 1993 to September 2008 (192,936 individuals) and included outpatient, inpatient, laboratory, and prescription claims. Laboratory data included all tests performed along with the results of those tests. Pharmacy data included all pertinent patient demographics and medication dosing information regardless of pharmacy location or affiliation. This database has been used previously and is a generally complete claims record for covered patients.24 Patient data were de-identified but patients could be linked across medical and prescription claims and years as long as the patient had coverage during these years. The University at Buffalo Institutional Review Board approved the study.
Study Cohorts and Patient Population
Two retrospective cohort studies were performed to evaluate the association between PPI prescription use and the development of the two outcomes of interest: AKI and CKD. Patients aged 18 years or older enrolled in the HMO during the study period were included in the study. All patients required at least 12 months of enrollment before the index date. Patients in the AKI cohort required at least 90 days of follow-up data, and patients in the CKD cohort required at least 12 months of follow-up data. Patients were excluded if they had evidence of pre-existing renal disease up to 12 months prior to the index date defined according to the presence of relevant International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) codes (Appendix 1). These inclusion and exclusion criteria assured that all patients had at least 12 months of claims data prior to renal disease onset. The index date for those exposed to PPIs was the date of first pharmacy prescription for these drugs and, for the nonexposed, the index date followed the first 12 months of claims.
Primary Exposure
The primary exposure was defined as a prescription claim for a PPI. Medications containing esomeprazole, lansoprazole, omeprazole, pantoprazole, or rabeprazole were counted as PPIs. The medications were identified based on National Drug Codes (NDCs), or brand, and/or generic names.
Outcomes
The primary outcome for the AKI cohort was defined as a documented ICD-9-CM code of 584.X or an estimated glomerular filtration rate (eGFR) of less than 60 ml/min/1.73 m2 within 90 days after the index date. The primary outcome for the CKD cohort was defined as an inpatient or outpatient visit with an ICD-9-CM code of 585.X or an eGFR of less than 60 ml/min/1.73 m2. End stage renal disease (ESRD) was also included as a CKD outcome, which was defined based on the presence of ICD-9-CM code 585.6 or documentation of receipt of dialysis (V45.11 or V56.X). All outcomes were ascertained from the time of cohort entry until last known follow-up within the database.
Covariates
Baseline covariates were ascertained up to 12 months prior to the index date for both the exposed and nonexposed groups. Covariates included age, sex, relevant comorbidities, and medication use. Comorbidities and medications were chosen based on their correlation with kidney disease, PPI use, or overall health status. As a proxy for health status, comorbidities from the Charlson comorbidity index (obesity, diabetes, hypertension, hyperlipidemia, metastatic cancer, osteoarthritis, rheumatoid arthritis, liver disease, Helicobacter pylori infection, heart failure, peripheral vascular disease, cerebrovascular disease, and human immunodeficiency virus (HIV) ) were included [Appendix 2].25 Exposure to medications included histamine-2 (H2)-receptor blockers, angiotensin-converting enzyme inhibitors (ACEs), angiotensin receptor blockers (ARBs), antibiotics, antivirals, nonsteroidal antiinflammatory drugs (NSAIDs), calcineurin inhibitors, and diuretics as evaluated using NDC codes and brand-generic names. All variables were extracted from inpatient, outpatient, and prescription claims data using appropriate ICD-9-CM, NDC, and brand-generic names. Use of over-the-counter (OTC) medications, including PPIs and H2-receptor blockers, was not captured in this claims database.
Statistical Analysis
Baseline characteristics between groups were compared with the Student t test for continuous variables and the Χ2-test for categorical variables. Incidence rates per 1000 person-years were computed for outcomes, and confidence intervals (CI) were estimated based on normal distributions. Logistic regression models were used to estimate the odds ratios (ORs) and 95% CIs for the association between PPI exposure and risk of renal outcomes (AKI and CKD). Each potential confounding factor was examined individually using a change-in-estimate criterion, and considered covariates as confounders if they changed the OR of interest by 10% or more.26 For each identified confounder, separate 3-covariate logistic regression models examining the exposures’ effects on the outcome were run, adjusted individually for each confounder. Then a multivariable logistic regression model including all identified confounders was run to estimate the fully modeled adjusted ORs of interest. A 2-tailed p value less than 0.05 was deemed statistically significant, and all analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Sensitivity Analyses
Additional analyses were performed to further explore the possibility of residual confounding factors. First, the risk of AKI and CKD among PPI users using H2-receptor blockers as a negative control and active comparator was assessed, where medications that contained ranitidine, cimetidine, famotidine, or nizatidine were classified as H2-receptor blockers based on their NDC or brand-generic name. In this analysis, the date of first pharmacy prescription for a PPI or H2-receptor blocker was used as the index date. Second, the risk of renal outcomes in a 1:1 propensity score-matched cohort was examined. Propensity scores were calculated for each cohort using nonparsimonious logistic regression models with PPI exposure as the dependent variable, and predictor variables of age, gender, medical conditions (diabetes, cerebrovascular disease, heart failure, HIV, liver disease, metastatic cancer, obesity, osteoarthritis, rheumatoid arthritis, hypertension, hyperlipidemia, H. pylori infection, and peripheral vascular disease), and medication use (antibiotics, amphotericin B, antivirals, antiretrovirals, mesalamine, NSAIDs, ACE inhibitors, ARBs, diuretics, calcineurin inhibitors, and H2-receptor blockers). Nearest-neighbor matching without replacement was used, with a caliper distance set as 0.2. Conditional logistic regression models were used to examine the association between PPI and outcomes. Any variables that were not balanced following matching were included in the adjusted models.
Results
There were 93,335 patients in the AKI cohort and 84,600 patients in the CKD cohort. Cohort selection is detailed in Figure 1. Baseline characteristics of the AKI and CKD cohorts are described in Tables 1 and 2, respectively. The median follow-up was 6.8 years in the CKD cohort and 90 days in the AKI cohort. In both cohorts, PPI users were older at baseline and had higher prevalence of comorbidities compared to nonusers. PPI users also more frequently took an antibiotic, antiviral, NSAID, ACE inhibitor, ARB, diuretic, calcineurin inhibitor, or H2-receptor blocker in the 12 months prior to the index date. The frequency of AKI and CKD events based on age and eGFR are described in Table 3. In both cohorts, the frequency of events were highest among those 65 years of age or older with a mild to moderate decrease in eGFR (range, 45–59 ml/min/1.73 m2) reported most frequently.
Figure 1.
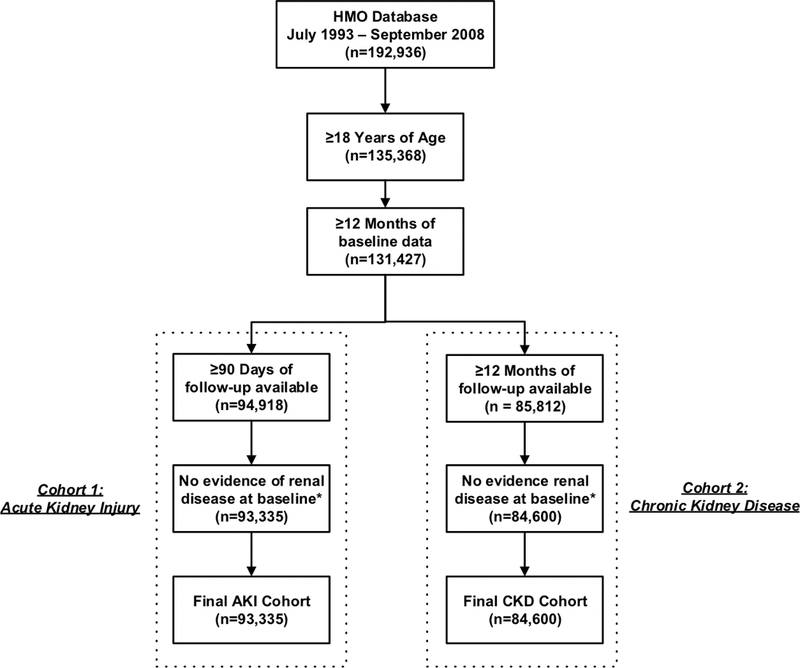
Flow chart of cohort assembly within the acute kidney injury and chronic kidney disease cohorts (Abb. AKI, acute kidney injury; CKD, chronic kidney disease; HMO, health maintenance organization). aPatients were excluded if they had evidence of pre-existing renal disease up to 12 months prior to the index date defined based on the presence of relevant ICD-9-CM codes (Appendix2)
Table 1.
Baseline characteristics of new PPI users within the AKI cohort and the propensity-matched AKI cohort
| AKI Cohort | Propensity-Matched AKI Cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic, n (%) | All patients (n = 93,335) |
PPI (n = 16,593) |
No PPI (n = 76,742) |
p value | All Patients (n = 27,778) |
PPI (n = 13,889) |
No PPI (n = 13,889) |
p value |
| Age, years, mean (SD) | 44.1 (16.7) | 53.2 (17.4) | 42.1 (15.9) | <0.0001 | 51.1 (17.0) | 51.4 (17.2) | 50.9 (16.8) | 0.017 |
| Gender | ||||||||
| Female | 55,676 (59.6) | 10,376 (62.5) | 45,300 (59.0) | <0.0001 | 17,036 (61.3) | 8551 (61.6) | 8485 (61.1) | 0.44 |
| Medical Conditions | ||||||||
| Diabetes | 4842 (5.19) | 1810 (10.9) | 3032 (3.95) | <0.0001 | 2547 (9.17) | 1292 (9.30) | 1255 (9.04) | 0.44 |
| CVD | 1520 (1.63) | 878 (5.29) | 642 (0.84) | <0.0001 | 999 (3.60) | 533 (3.84) | 466 (3.36) | 0.031 |
| Heart Failure | 1224 (1.31) | 782 (4.71) | 442 (0.58) | <0.0001 | 792 (2.85) | 442 (3.18) | 350 (2.52) | 0.0009 |
| HIV | 43 (0.05) | 15 (0.09) | 28 (0.04) | 0.0033 | 19 (0.07) | 11 (0.08) | 8 (0.06) | 0.49 |
| Liver Disease | 263 (0.28) | 134 (0.81) | 129 (0.17) | <0.0001 | 174 (0.63) | 89 (0.64) | 85 (0.61) | 0.76 |
| Metastatic Cancer | 339 (0.36) | 208 (1.25) | 131 (0.17) | <0.0001 | 245 (0.88) | 127 (0.91) | 118 (0.85) | 0.56 |
| Obesity | 1734 (1.86) | 480 (2.89) | 1254 (1.63) | <0.0001 | 761 (2.74) | 361 (2.60) | 400 (2.88) | 0.15 |
| Osteoarthritis | 3081 (3.30) | 1365 (8.23) | 1716 (2.24) | <0.0001 | 1842 (6.63) | 949 (6.83) | 893 (6.43) | 0.18 |
| Rheumatoid Arthritis | 782 (0.84) | 400 (2.41) | 382 (0.50) | <0.0001 | 505 (1.82) | 259 (1.86) | 246 (1.77) | 0.56 |
| Hypertension | 13,316 (14.3) | 4536 (27.3) | 8780 (11.4) | <0.0001 | 6973 (25.1) | 3411 (24.6) | 3562 (25.7) | 0.037 |
| Hyperlipidemia | 7539 (8.08) | 2793 (16.8) | 4746 (6.18) | <0.0001 | 4468 (16.1) | 2104 (15.2) | 2364 (17.0) | <0.0001 |
| H. pylori | 77 (0.08) | 67 (0.40) | 10 (0.01) | <0.0001 | 35 (0.13) | 25 (0.18) | 10 (0.07) | 0.011 |
| PVD | 479 (0.51) | 242 (1.46) | 237 (0.31) | <0.0001 | 299 (1.08) | 161 (1.16) | 138 (0.99) | 0.18 |
| Medications | ||||||||
| Aminoglycoside | 12 (0.01) | 9 (0.05) | 3 (0.00) | <0.0001 | 6 (0.02) | 3 (0.02) | 3 (0.02) | 0.66 |
| Cephalosporin | 6563 (7.03) | 1890 (11.4) | 4673 (6.09) | <0.0001 | 2929 (10.5) | 1434 (10.3) | 1495 (10.8) | 0.23 |
| Fluoroquinolone | 3939 (4.22) | 1907 (11.5) | 2032 (2.65) | <0.0001 | 2524 (9.09) | 1257 (9.05) | 1267 (9.12) | 0.83 |
| Macrolide | 9100 (9.75) | 3111 (18.8) | 5989 (7.80) | <0.0001 | 4742 (17.1) | 2307 (16.6) | 2435 (17.5) | 0.041 |
| Penicillin | 18,009 (19.3) | 4562 (27.5) | 13,447 (17.5) | <0.0001 | 7193 (25.9) | 3574 (25.7) | 3619 (26.1) | 0.54 |
| Penicillinase-resistant | 353 (0.38) | 52 (0.31) | 301 (0.39) | 0.13 | 86 (0.31) | 50 (0.36) | 36 (0.26) | 0.13 |
| Sulfa | 6026 (6.46) | 1733 (10.4) | 4293 (5.59) | <0.0001 | 2691 (9.69) | 1319 (9.50) | 172 (9.88) | 0.28 |
| Tetracycline | 1177 (1.26) | 305 (1.84) | 872 (1.14) | <0.0001 | 395 (1.42) | 220 (1.58) | 175 (1.26) | 0.023 |
| Vancomycin | 25 (0.03) | 14 (0.08) | 11 (0.01) | <0.0001 | 14 (0.05) | 8 (0.06) | 6 (0.04) | 0.59 |
| Nitrofurantoin | 1177 (1.26) | 355 (2.14) | 822 (1.07) | <0.0001 | 513 (1.85) | 257 (1.85) | 256 (1.84) | 0.96 |
| Amphotericin B | 11 (0.01) | 7 (0.04) | 4 (0.01) | 0.0009 | 8 (0.03) | 5 (0.04) | 3 (0.02) | 0.73 |
| Antiviral | 1179 (1.26) | 377 (2.27) | 802 (1.05) | <0.0001 | 573 (2.06) | 283 (2.04) | 290 (2.09) | 0.77 |
| Antiretroviral | 9 (0.01) | 4 (0.02) | 5 (0.01) | 0.059 | 4 (0.01) | 2 (0.01) | 2 (0.01) | 1.0 |
| Mesalamine | 173 (0.19) | 95 (0.57) | 78 (0.10) | <0.0001 | 118 (0.42) | 63 (0.45) | 55 (0.40) | 0.46 |
| NSAID | 19,396 (20.8) | 5894 (35.5) | 13,502 (17.6) | <0.0001 | 9223 (33.2) | 4505 (32.4) | 4718 (34.0) | 0.0067 |
| ARB | 954 (1.02) | 587 (3.54) | 367 (0.48) | <0.0001 | 662 (2.38) | 352 (2.53) | 310 (2.23) | 0.099 |
| ACE Inhibitor | 6748 (7.23) | 3026 (18.2) | 3722 (4.85) | <0.0001 | 4346 (15.7) | 2143 (15.4) | 2203 (15.9) | 0.32 |
| Diuretic | 7749 (8.30) | 3545 (21.4) | 4204 (5.48) | <0.0001 | 4957 (17.9) | 2439 (17.6) | 2518 (18.1) | 0.22 |
| Calcineurin Inhibitor | 24 (0.03) | 15 (0.09) | 9 (0.01) | <0.0001 | 15 (0.05) | 9 (0.06) | 6 (0.04) | 0.44 |
| H2-Blocker | 6226 (6.67) | 4301 (25.9) | 1925 (2.51) | <0.0001 | 4230 (15.2) | 2328 (16.8) | 1902 (13.7) | <0.0001 |
Abbreviations: ACE inhibitor, angiotensin converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin receptor blocker; CVD, cerebrovascular disease; H2-blocker, histamine-2 receptor blocker; HIV, human immunodeficiency virus; H. pylori, Helicobacter pylori infection; NSAID, non-steroidal anti-inflammatory drug; PPI, proton pump inhibitors; PVD, peripheral vascular disease; SD, standard deviation
Table 2.
Baseline characteristics of new PPI users within the CKD cohort and the propensity-matched CKD cohort
| CKD Cohort | Propensity-Matched CKD Cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic, n (%) | All patients (n = 84,600) |
PPI (n = 14,514) |
No PPI (n = 70,086) |
p value | All Patients (n = 24,186) |
PPI (n = 12,093) |
No PPI (n = 12,093) |
p value |
| Age, years, mean (SD) | 44.2 (16.7) | 53.4 (17.2) | 42.4 (16.0) | <0.0001 | 51.6 (16.9) | 51.6 (17.0) | 51.6 (16.8) | 0.90 |
| Gender | ||||||||
| Female | 50,442 (59.6) | 9115 (62.8) | 41,327 (59.0) | <0.0001 | 14,853 (61.4) | 7457 (61.7) | 7396 (61.2) | 0.42 |
| Medical Conditions | ||||||||
| Diabetes | 4381 (5.18) | 1575 (10.9) | 2806 (4.00) | <0.0001 | 2275 (9.41) | 1160 (9.59) | 1115 (9.22) | 0.32 |
| CVD | 1351 (1.60) | 756 (5.21) | 595 (0.85) | <0.0001 | 879 (3.63) | 455 (3.76) | 424 (3.51) | 0.29 |
| Heart Failure | 1075 (1.27) | 665 (4.58) | 410 (0.58) | <0.0001 | 706 (2.92) | 379 (3.13) | 327 (2.70) | 0.047 |
| HIV | 39 (0.05) | 15 (0.10) | 24 (0.03) | 0.0004 | 21 (0.09) | 9 (0.07) | 12 (0.10) | 0.51 |
| Liver Disease | 207 (0.24) | 102 (0.70) | 105 (0.15) | <0.0001 | 132 (0.55) | 72 (0.60) | 60 (0.50) | 0.29 |
| Metastatic Cancer | 261 (0.31) | 141 (0.97) | 120 (0.17) | <0.0001 | 175 (0.72) | 87 (0.72) | 88 (0.73) | 0.94 |
| Obesity | 1587 (1.88) | 428 (2.95) | 1159 (1.65) | <0.0001 | 665 (2.75) | 329 (2.72) | 336 (2.78) | 0.78 |
| Osteoarthritis | 2833 (3.35) | 1224 (8.43) | 1609 (2.30) | <0.0001 | 1657 (6.85) | 834 (6.90) | 823 (6.81) | 0.78 |
| Rheumatoid Arthritis | 707 (0.84) | 354 (2.44) | 353 (0.50) | <0.0001 | 445 (1.84) | 229 (1.89) | 216 (1.79) | 0.53 |
| Hypertension | 12,186 (14.4) | 4019 (27.7) | 8167 (11.7) | <0.0001 | 6230 (25.8) | 3014 (24.9) | 3216 (26.6) | 0.003 |
| Hyperlipidemia | 6789 (8.02) | 2511 (17.3) | 4278 (6.10) | <0.0001 | 3992 (16.5) | 1896 (15.7) | 2096 (17.3) | 0.0005 |
| H. pylori | 65 (0.08) | 59 (0.41) | 6 (0.01) | <0.0001 | 21 (0.09) | 15 (0.12) | 6 (0.05) | 0.049 |
| PVD | 428 (0.51) | 207 (1.43) | 221 (0.32) | <0.0001 | 258 (1.07) | 134 (1.11) | 124 (1.03) | 0.53 |
| Medications | ||||||||
| Aminoglycoside | 9 (0.01) | 6 (0.04) | 3 (0.00) | 0.0013 | 6 (0.02) | 3 (0.02) | 3 (0.02) | 0.66 |
| Cephalosporin | 6022 (7.12) | 1670 (11.5) | 4352 (6.21) | <0.0001 | 2560 (10.6) | 1260 (10.4) | 1300 (10.8) | 0.40 |
| Fluoroquinolone | 3394 (4.01) | 1607 (11.1) | 1787 (2.55) | <0.0001 | 2134 (8.82) | 1082 (8.95) | 1052 (8.70) | 0.49 |
| Macrolide | 8206 (9.70) | 2727 (18.8) | 5479 (7.82) | <0.0001 | 4124 (17.1) | 2024 (16.7) | 2100 (17.4) | 0.19 |
| Penicillin | 16,659 (19.7) | 4098 (28.2) | 12,561 (17.9) | <0.0001 | 6404 (26.5) | 3146 (26.0) | 3258 (26.9) | 0.10 |
| Penicillinase-resistant | 337 (0.40) | 49 (0.34) | 288 (0.41) | 0.20 | 78 (0.32) | 43 (0.36) | 35 (0.29) | 0.36 |
| Sulfa | 5690 (6.73) | 1603 (11.0) | 4087 (5.83) | <0.0001 | 2436 (10.07) | 1203 (9.95) | 1233 (10.2) | 0.52 |
| Tetracycline | 1100 (1.30) | 281 (1.94) | 819 (1.17) | <0.0001 | 391 (1.62) | 211 (1.74) | 180 (1.49) | 0.11 |
| Vancomycin | 24 (0.03) | 13 (0.09) | 11 (0.02) | <0.0001 | 12 (0.05) | 5 (0.04) | 7 (0.06) | 0.56 |
| Nitrofurantoin | 1070 (1.26) | 312 (2.15) | 758 (1.08) | <0.0001 | 447 (1.85) | 230 (1.90) | 217 (1.79) | 0.53 |
| Amphotericin B | 7 (0.01) | 5 (0.03) | 2 (0.00) | 0.0023 | 3 (0.01) | 1 (0.01) | 2 (0.02) | 1.0 |
| Antiviral | 1051 (1.24) | 321 (2.21) | 730 (1.04) | <0.0001 | 464 (1.92) | 221 (1.83) | 243 (2.01) | 0.30 |
| Antiretroviral | 6 (0.01) | 2 (0.01) | 4 (0.01) | 0.28 | 3 (0.01) | 1 (0.01) | 2 (0.02) | 1.0 |
| Mesalamine | 159 (0.19) | 89 (0.61) | 70 (0.10) | <0.0001 | 104 (0.43) | 54 (0.45) | 50 (0.41) | 0.69 |
| NSAID | 17,915 (21.2) | 5270 (36.3) | 12,645 (18.0) | <0.0001 | 8183 (33.8) | 4029 (33.3) | 4154 (34.4) | 0.089 |
| ARB | 808 (0.96) | 500 (3.44) | 308 (0.44) | <0.0001 | 572 (2.37) | 302 (2.50) | 270 (2.23) | 0.18 |
| ACE Inhibitor | 6102 (7.21) | 2638 (18.2) | 3464 (4.94) | <0.0001 | 3826 (15.8) | 1895 (15.7) | 1931 (16.0) | 0.52 |
| Diuretic | 7004 (8.28) | 3088 (21.3) | 3916 (5.59) | <0.0001 | 4385 (18.1) | 2152 (17.8) | 2233 (18.5) | 0.18 |
| Calcineurin Inhibitor | 21 (0.02) | 13 (0.09) | 8 (0.01) | <0.0001 | 12 (0.05) | 6 (0.05) | 6 (0.05) | 1.0 |
| H2-Blocker | 5896 (6.97) | 4066 (28.0) | 1830 (2.61) | <0.0001 | 4044 (16.7) | 2236 (18.5) | 1808 (15.0) | <0.0001 |
Abbreviations: ACE inhibitor, angiotensin converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin receptor blocker; CVD, cerebrovascular disease; H2-blocker, histamine-2 receptor blocker; HIV, human immunodeficiency virus; H. pylori, Helicobacter pylori infection; NSAID, nonsteroidal antiinflammatory drug; PPI, proton pump inhibitors; PVD, peripheral vascular disease; SD, standard deviation
Table 3.
Frequency of acute kidney injury and chronic kidney disease stratified by age and GFR categories
| Acute Kidney Injurya | Chronic Kidney Diseaseb | |||||||
|---|---|---|---|---|---|---|---|---|
| Age (years) | 18–39 | 40–64 | ≥65 | 18–39 | 40–64 | ≥65 | ||
| No. of events (%) | 7 | 72 | 104 | 418 | 2410 | 3162 | ||
| GFR Categoriesc
(ml/min/1.73 m2) |
Description | eGFR Range | ||||||
| Mildly to moderately decreased | 45–59 | 6 (86) | 62 (86) | 80 (77) | 382 (91) | 2163 (89) | 2491 (79) | |
| Moderately to severely decreased | 30–44 | 1 (14) | 9 (13) | 19 (18) | 25 (6) | 217 (9) | 576 (18) | |
| Severely decreased | 15–29 | 0 | 1 (1) | 4 (4) | 9 (2.2) | 27 (1.1) | 92 (2.9) | |
| Kidney failure | <15 | 0 | 0 | 1 (1) | 2 (0.48) | 3 (0.12) | 3 (0.1) | |
Abbreviations: eGFR, estimated glomerular filtration rate; AKI, acute kidney injury; CKD, chronic kidney disease; KDIGO, Kidney Disease Improving Global Outcomes
Data are presented as n (%)
This includes 183 AKI events identified based on the estimated GFR of the total 215 AKI events
This includes 5036 CKD events identified based on the estimated GFR of the total 6871 CKD events
GFR categories are based on the CKD nomenclature within the KDIGO Clinical Practice Guidelines27
Association between PPI Use and Risk of AKI
In the AKI cohort, there were 148 events (0.89%) among 16,593 patients exposed to PPIs and 67 events (0.09%) among 76,742 unexposed patients (Table 4). The incidence rate of AKI was higher in the PPI group than among nonusers (36.4 vs. 3.54 per 1000 person-years, p<0.0001, respectively). In the model adjusted for age, diabetes, heart failure, hypertension, fluoroquinolone use, ACE inhibitor use, ARB use, diuretic use, and H2-receptor blocker use, PPI use was associated with a significantly increased risk of AKI (adjusted (a)OR 4.35; 95% CI 3.14–6.04; p<0.0001).
Table 4.
Incidence rates and odds ratios for PPI use and risk of acute kidney injury
| AKI Cohort | Propensity-Matched AKI Cohort | |||
|---|---|---|---|---|
|
PPI Users (n = 16,593) |
PPI Nonusers (n = 76,742) |
PPI Users (n = 13,889) |
PPI Nonusers (n = 13,889) |
|
| Number of events (%) | 148 (0.89) | 67 (0.09) | 115 (0.83) | 29 (0.21) |
| Incident rate per 1000 person-years | 36.4 | 3.54 | 33.7 | 8.48 |
|
Odds Ratio (95% CI) |
p value |
Odds Ratio (95% CI) |
p value |
|
| Unadjusted PPI use vs. no PPI use | 10.3 (7.71 – 13.8) | <0.0001 | 3.99 (2.65 – 6.00) | <0.0001 |
| PPI use vs. no PPI use | 4.35 (3.14 – 6.04) a | <0.0001 | 3.93 (2.61– 5.93)b | <0.0001 |
Abbreviations: AKI, acute kidney injury; PPI, proton pump inhibitors; CI, confidence interval; ACE, angiotensin-converting enzyme; ARBs, angiotensin II receptor blockers; H2, histamine-2; NSAIDs, nonsteroidal anti-inflammatory drugs
Adjusted for age, diabetes, heart failure, hypertension, and use of fluoroquinolones, ACE inhibitors, ARBs, diuretics, and H2-receptor blockers
Adjusted for age, cerebrovascular disease, heart failure, hypertension, hyperlipidemia, and use of macrolides, tetracyclines, NSAIDs, and H2-receptor blockers
Association between PPI Use and Risk of CKD
In the CKD cohort, there were 2370 events (15.3%) among 14,514 PPI users and 4501 (6.42%) events among 70,086 nonusers (Table 5). The incidence rate of CKD was significantly higher in patients exposed to PPIs compared to non-PPI users (34.3 vs. 8.75 per 1000 person-years, p<0.0001, respectively). In the model adjusted for age, diabetes, hypertension, hyperlipidemia, ACE inhibitor use, diuretic use, and H2-receptor blocker use, PPI use was associated with a 1.2-times risk of incident CKD relative to nonusers (aOR 1.20; 95% CI 1.12–1.28; p<0.0001).
Table 5.
Incidence rates and odds ratios for PPI use and risk of chronic kidney disease
| CKD Cohort | Propensity-Matched CKD Cohort | |||
|---|---|---|---|---|
|
PPI Users (n = 14,514) |
PPI Nonusers (n = 70,086) |
PPI Users (n = 12,093) |
PPI Nonusers (n = 12,093) |
|
| Number of events (%) | 2370 (15.3) | 4501 (6.42) | 1710 (14.1) | 1500 (12.4) |
| Incidence rate per 1000 person-years | 34.3 | 8.75 | 30.5 | 16.5 |
|
Odds Ratio (95% CI) |
p value |
Odds Ratio (95% CI) |
p value |
|
| Unadjusted PPI use vs. no PPI use | 2.84 (2.70 – 3.00) | <0.0001 | 1.16 (1.08 – 1.25) | <0.0001 |
| PPI use vs. no PPI use | 1.20 (1.12 – 1.28) a | <0.0001 | 1.20 (1.11 – 1.29) b | <0.0001 |
Abbreviations: CKD, chronic kidney disease; PPI, proton pump inhibitor; CI, confidence interval; ACE, angiotensin-converting enzyme; ARBs, angiotensin II receptor blockers; H2, histamine-2
Adjusted for age, diabetes, hypertension, hyperlipidemia, and use of ACE inhibitors, diuretics, and H2-receptor blockers
Adjusted for heart failure, hypertension, hyperlipidemia, H. pylori infection, and use of H2-receptor blockers
Sensitivity Analyses
Results of our first sensitivity analyses examining the risk of AKI and CKD associated with PPIs at baseline using H2-receptor blockers as a negative control and active comparator are displayed in Table 6. There were 89,524 patients in the AKI cohort, of which 9736 were exposed to PPIs and 11,397 were exposed to H2-receptor blockers. Patients receiving both a PPI and an H2-receptor blocker on the index date were excluded (n=82). The incidence rate of AKI among PPI users was higher than controls (46.5 vs. 3.74 per 1000 person-years, p<0.0001) and H2-receptor blocker users (46.5 vs. 9.26 per 1000 person-years, p<0.0001), respectively. PPI use was associated with an increased risk of AKI compared to non-PPI users (aOR 4.31; 95% CI 3.05–6.09; p<0.0001), consistent with the primary analysis. PPI use was also associated with an increased risk of AKI when compared directly to H2-receptor blocker use (aOR 3.78; 95% CI 2.44–5.84; p<0.0001). After adjusting for confounders, H2-receptor blockers were not associated with an increased risk of AKI (aOR 1.16; 95% CI 0.72–1.85; p=0.55).
Table 6.
PPI use and risk 00of incident acute kidney injury or chronic kidney disease – sensitivity analyses
| AKI Cohort | CKD Cohort | |||||
|---|---|---|---|---|---|---|
|
PPI Users (n = 9736) |
H2-Receptor Blocker Users (n = 11,397) |
Nonusers (n = 68,391) |
PPI Users (n = 8192) |
H2-Receptor Blocker Users (n = 10,843) |
Nonusers (n = 61,874) |
|
| Number of events (%) | 111 (1.14) | 26 (0.23) | 63 (0.09) | 1159 (14.2) | 1572 (14.5) | 3638 (5.88) |
| Incident rate per 1000 person-years | 46.5 | 9.26 | 3.74 | 34.0 | 21.0 | 8.32 |
|
Odds Ratio (95% CI) |
p value |
Odds Ratio (95% CI) |
p value |
|||
| Unadjusted PPI use vs. no PPI use | 12.6 (9.25 – 17.2) | <0.0001 | 2.63 (2.45 – 2.82) | <0.0001 | ||
| PPI use vs. no PPI use | 4.31 (3.05 – 6.09) † | <0.0001 | 1.18 (1.09– 1.28) ‡ | <0.0001 | ||
| Unadjusted H2-receptor blocker use vs. no H2-receptor blocker use | 2.50 (1.58 – 3.95) | <0.0001 | 2.71 (2.54 – 2.88) | <0.0001 | ||
| H2-receptor blocker use vs. no H2-receptor blocker use | 1.16 (0.72 – 1.85) † | 0.55 | 1.49 (1.39 – 1.60) ‡ | <0.0001 | ||
| Unadjusted PPI use vs. H2-receptor blocker use | 5.05 (3.29 – 7.75) | <0.0001 | 0.97 (0.89 – 1.05) | 0.48 | ||
| PPI use vs. H2-receptor blocker use | 3.78 (2.44 – 5.84) a | <0.0001 | 0.81 (0.74 – 0.89) b | <0.0001 | ||
Abbreviations: CKD, chronic kidney disease; AKI, acute kidney injury; PPI, proton pump inhibitor; CI, confidence interval; ACE, angiotensin-converting enzyme; ARBs, angiotensin II receptor blockers; H2, histamine-2
Adjusted for adjusted for age, diabetes, cerebrovascular disease, heart failure, hypertension, hyperlipidemia, and use of fluoroquinolones, ACE inhibitors, ARBs, and diuretics
Adjusted for age, diabetes, hypertension, hyperlipidemia, and use of ACE inhibitors, and diuretics
In the CKD cohort, 80,909 patients were included with 8192 exposed to PPIs and 10,843 exposed to H2-receptor blockers. Sixty-six patients taking both a PPI and H2-receptor blocker on the index date were excluded. The incidence rate of CKD was higher among PPI users than controls (34.0 vs. 8.32 per 1000 person-years, p<0.0001) and H2-receptor blocker users (34.0 vs. 21.0 per 1000 person-years, p<0.0001), respectively. The association between risk of CKD among PPI users compared to that of nonusers (aOR 1.18; 95% CI 1.09–1.28; p<0.0001) was similar to the primary analysis. Use of H2-receptor blockers at baseline was associated with an increased risk of CKD (aOR 1.49; 95% CI 1.39–1.60; p<0.0001) and, when comparing PPI users to H2-receptor blocker users, the risk of CKD was similar between groups in unadjusted models (OR 0.97; 95% CI 0.89–1.05; p=0.48) and decreased in adjusted models (aOR 0.81; 95% CI 0.74–0.89; p<0.0001).
Baseline characteristics of the propensity-matched AKI and CKD cohorts are described in Tables 1 and 2. Results were consistent with the primary analyses (Tables 4 and 5). PPI use was associated with an increased incidence of AKI compared with nonusers (33.7 vs. 8.48 per 1000 person-years, p<0.0001, respectively) and an increased risk of AKI (OR 3.93; 95% CI 2.61–5.93; p<0.0001). The PPI users had an increased incidence of CKD compared to nonusers (30.5 vs. 16.5 per 1000 person-years, p<0.0001, respectively), and PPI use was associated with an increased risk of CKD (OR 1.20; 95% CI 1.11–1.29; p<0.0001), consistent with adjusted models in the primary analysis (Table 5).
Discussion
In this longitudinal, retrospective cohort study of more than 190,000 patients, baseline PPI use was independently associated with a 20% higher risk of incident CKD after adjusting for demographics, comorbidities, and concomitant medications. There was a 4-fold increase in the risk of AKI among those exposed to PPIs. These results were consistent within a propensity-matched analysis and when directly compared with the use of H2-receptor blockers. Our results are in general agreement with previous results on this topic and strengthen existing evidence of an association between PPI exposure and the development of kidney disease.
These results expand on the findings of recent observational cohort studies. One study evaluated the relationship between PPIs and CKD in two cohorts: 10,482 participants in the Arthrosclerosis Risk in Communities study and 248,751 patients in the Geisinger Health System.19 In both cohorts, participants who used PPIs at baseline had a significantly increased risk of incident CKD compared with nonusers. Similarly, another study built a cohort of 173,321 new users of PPIs within the Department of Veterans Affairs national databases and followed these patients over 5 years.20 There was a relationship between PPI exposure and increased risk of incident CKD, CKD progression, and ESRD. Our study adds to the existing literature by describing an association between PPI use and incidence of CKD over a long period within an HMO database. The consistency of our findings with previous observations suggests the need to judiciously prescribe PPIs. Given the high prevalence of PPI use, overuse, and long-term adverse outcomes, a focus on PPI deprescribing is necessary to reduce PPI burden and harm.
Although these findings support a relationship between PPI exposure and incident AKI, the precision of the estimate (i.e., CI) is limited by the number of AKI cases. The CI ranged from 3.14 to 6.04, suggesting that PPIs could increase the odds of AKI development by as much as 6-fold. Of note, this relationship persisted within a propensity score-matched cohort, as the multivariable-adjusted OR for PPI exposure was 3.93 (95% CI 2.61–5.93). AKI in PPI users is likely to be underdiagnosed given that PPIs are often viewed as safe and well-tolerated medications. A heightened awareness among health care professionals of the adverse outcomes associated with PPIs is a necessary first step to modify PPI overutilization.
Our results add to a growing list of concerning side effects and adverse outcomes associated with PPIs including hypomagnesemia, vitamin B12 deficiency, fractures, pneumonia, and Clostridium difficile infection.11, 12, 27, 28 An increased risk of any side effect from these medications is concerning, especially considering that up to 70% of patients are taking PPIs without a valid indication.2 There is a clear need to decrease inappropriate PPI usage. To help achieve this goal, evidence-based clinical practice guidelines have been published to aid clinicians in deprescribing PPIs.9 Deprescribing may involve reducing the dosage, using “as needed” dosing, or stopping acid reduction therapy altogether in certain eligible patients. Although deprescribing is an essential part of best prescribing practices, several barriers exist in everyday practice. These include personal factors (i.e., maintaining relationships with patients and colleagues), sociocultural factors (i.e., medical culture of prescribing), and organizational factors (i.e., fast pace and competing demands of practice).29 Interventions focused on safer PPI prescribing are necessary and should consider these influences.
This study had several strengths. We included a large, representative sample of patients over 15 years. Our comprehensive data source allowed us to collect information on laboratory values in addition to medication usage and comorbidities, which allowed accurate assessment of outcomes, exposures, and confounders. The study yielded robust results supported by multiple sensitivity analyses, which showed that the findings may be generalizable.
A limitation, as with all observational studies, is the risk of residual confounding factors. To account for this, we adjusted for multiple confounders and performed several sensitivity analyses, including a propensity-matched cohort. However, several potential confounders could not be evaluated. Race was unspecified for a significant proportion of patients, so we were unable to include this variable in the study. In addition, we could not account for OTC PPI and H2-receptor blocker use, so it is possible that some patients who used OTC products were misclassified as nonusers. Of note, omeprazole was the first PPI to become available OTC in 2003, whereas H2-receptor blockers (cimetidine and famotidine) were available OTC from 1995.30 We were also unable to account for other factors that could contribute to the development of kidney injury on the index date, including volume depletion. Additionally, there were limitations related to the use of claims data in the study. We were unable to assess whether patients were actually taking the medications they had filled. This is of particular concern for patients who may have had prescriptions filled automatically, especially through mail order, though we did not have information on the type of pharmacy that dispensed the PPI. Finally, there may have been surveillance bias. Patients receiving PPI therapy may have had more frequent contact with the health care system, so may have been more likely to receive testing that would indicate AKI or CKD. This would have led to an overestimation of risk of renal outcomes in PPI-exposed patients compared to non-exposed patients.
Conclusion
In summary, PPIs are frequently used drugs that are independently associated with AKI and CKD. A focus on health care provider education and deprescribing initiatives will be necessary to raise awareness and reduce PPI overutilization. Further studies are necessary to confirm our findings and provide evidence of a causal relationship, as well as to fully determine the mechanism by which PPIs may cause renal injury.
Acknowledgements:
D.M.J. is supported by the National Institutes of Health/National Heart, Lung, and Blood Institutes Loan Repayment Program (1 L30 HL138791–01). This work was supported in part by the National Center for Advancing Translational Sciences award UL1 TR001412 to the University at Buffalo.
Appendix 1. Pre-existing renal disease to be excluded
| Disease | ICD-9-CM |
|---|---|
| Hypertensive renal disease | 403.XX |
| Acute glomerulonephritis | 580.XX |
| Nephrotic syndrome | 581.XX |
| Chronic glomerulonephritis | 582.XX |
| Nephritis and nephropathy | 583.XX |
| Acute renal failure | 584.XX |
| Chronic renal failure | 585.XX |
| Renal failure, unspecified | 586.XX |
| Impaired renal function disease not elsewhere classifiable | 588.89 |
| Unspecified disorder of kidney and ureter | 593.9 |
| Kidney transplant | V42.0 |
| Dialysis | V45.1, V56.X |
Appendix 2. Identification of variables
| Covariate | ICD-9-CM |
|---|---|
| Obesity | 278.0 |
| Diabetes | 250.xx |
| Hypertension | 401.0, 401.1, 401.9 |
| Hyperlipidemia | 272.0, 272.1, 272.2, 272.4 |
| Metastatic cancer | 196.x-199.x |
| Osteoarthritis | 715.xx |
| Rheumatoid arthritis | 714.xx |
| Liver disease | 571.xx |
| H. pylori infection | 041.86 |
| Heart failure | 428 |
| Peripheral vascular disease | 443.9 |
| Cerebrovascular disease | 430–438 |
| HIV | 042–044.9 |
Footnotes
Conflict of Interest: All authors report no conflicts of interest
References
- 1.Abraham NS. Proton pump inhibitors: potential adverse effects. Curr Opin Gastroenterol 2012;6:615–20. [DOI] [PubMed] [Google Scholar]
- 2.Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ 2008;7634:2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;3:308–28; quiz 29. [DOI] [PubMed] [Google Scholar]
- 4.Ramakrishnan K, Salinas RC. Peptic ulcer disease. Am Fam Physician 2007;7:1005–12. [PubMed] [Google Scholar]
- 5.Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol 2012;4:219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batuwitage BT, Kingham JG, Morgan NE, Bartlett RL. Inappropriate prescribing of proton pump inhibitors in primary care. Postgrad Med J 2007;975:66–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol 2000;11:3118–22. [DOI] [PubMed] [Google Scholar]
- 8.Naunton M, Peterson GM, Bleasel MD. Overuse of proton pump inhibitors. J Clin Pharm Ther 2000;5:333–40. [DOI] [PubMed] [Google Scholar]
- 9.Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors: Evidence-based clinical practice guideline. Can Fam Physician 2017;5:354–64. [PMC free article] [PubMed] [Google Scholar]
- 10.Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf 2014;1:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benmassaoud A, McDonald EG, Lee TC. Potential harms of proton pump inhibitor therapy: rare adverse effects of commonly used drugs. CMAJ 2016;9:657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnstone J, Nerenberg K, Loeb M. Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Aliment Pharmacol Ther 2010;11:1165–77. [DOI] [PubMed] [Google Scholar]
- 13.Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol 2007;9:2047–56; quiz 57. [DOI] [PubMed] [Google Scholar]
- 14.Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int 2014;4:837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Risk of death among users of Proton Pump Inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open 2017;6:e015735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 2012;5:442–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harmark L, van der Wiel HE, de Groot MC, van Grootheest AC. Proton pump inhibitor-induced acute interstitial nephritis. Br J Clin Pharmacol 2007;6:819–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamal F, Khan MA, Molnar MZ, Howden CW. The Association Between Proton Pump Inhibitor Use With Acute Kidney Injury and Chronic Kidney Disease. J Clin Gastroenterol 2018;6:468–76. [DOI] [PubMed] [Google Scholar]
- 19.Lazarus B, Chen Y, Wilson FP, et al. Proton Pump Inhibitor Use and the Risk of Chronic Kidney Disease. JAMA Intern Med 2016;2:238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie Y, Bowe B, Li T, Xian H, Balasubramanian S, Al-Aly Z. Proton Pump Inhibitors and Risk of Incident CKD and Progression to ESRD. J Am Soc Nephrol 2016;10:3153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antoniou T, Macdonald EM, Hollands S, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open 2015;2:E166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu T, Zhou J, Zhang C. Acid-suppressive drugs and risk of kidney disease: A systematic review and meta-analysis. J Gastroenterol Hepatol 2018. April 12. doi: 10.1111/jgh.14157 [Epub ahead of print, April 12, 2018]. [DOI] [PubMed] [Google Scholar]
- 23.Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrol 2013;14:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs DM, Stefanovic F, Wilton G, Gomez-Caminero A, Schentag JJ. An integrated epidemiological and neural net model of the warfarin effect in managed care patients. Clin Pharmacol 2017;55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;5:373–83. [DOI] [PubMed] [Google Scholar]
- 26.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol 1989;1:125–37. [DOI] [PubMed] [Google Scholar]
- 27.Dial S, Alrasadi K, Manoukian C, Huang A, Menzies D. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ 2004;1:33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006;24:2947–53. [DOI] [PubMed] [Google Scholar]
- 29.Wallis KA, Andrews A, Henderson M. Swimming Against the Tide: Primary Care Physicians’ Views on Deprescribing in Everyday Practice. Ann Fam Med 2017;4:341–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drugs@FDA: FDA Approved Drug Products. Omeprazole. Available at https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021229 Accessed October 1, 2018.
- 31.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150. [Google Scholar]


