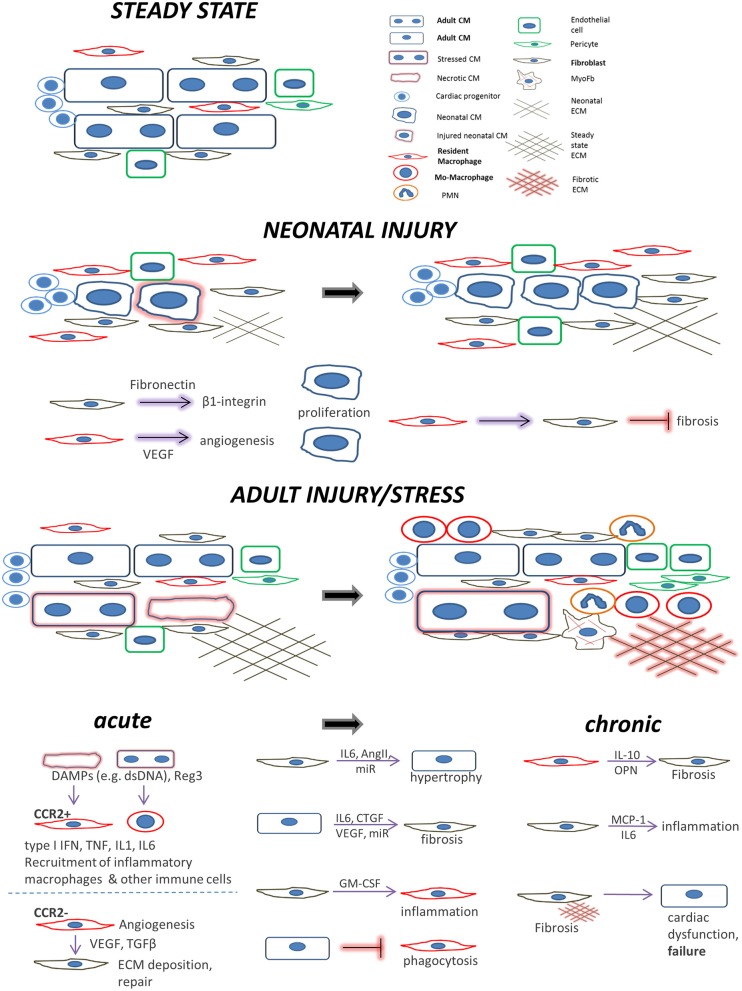
Figure 1.
Main cell players during cardiac repair and regeneration: the interplay between cardiomyocytes, fibroblasts, and macrophages. Healthy adult steady state: Quiescent cardiomyocytes are in a homeostatic balance with interspersed cardiac fibroblasts, resident macrophages, and endothelial cells. Neonatal injury: cardiac regeneration happens only within a very short window postpartum. If injured, neonatal mammalian cardiomyocytes can proliferate in a permissive environment of appropriate stiffness. This is achieved with the help of (a) neonatal cardiac fibroblasts (which secrete factors to induce cardiomyocyte proliferation via integrin signaling) and (b), resident, yolk-sac derived, CCR2− macrophages which secrete proangiogenic factors or instruct fibroblasts to assume alternative, less fibrogenic states following an injury. Adult injury: almost no regeneration occurs in the adult myocardium. Under acute injury conditions (myocardial infarction, infection, intrinsic cardiomyocyte defects, etc.) dying cardiomyocytes release danger signals (DAMPs) leading to mobilization and recruitment of monocytes and generation of monocyte-derived macrophages. Additional cardiomyocyte derived mediators such as the Reg3 proteins contribute to inflammatory macrophage expansion. CCR2+ resident macrophages recruit more monocyte-derived inflammatory macrophages and secrete agents (e.g., type I IFN, IL-1, TNF) propagating inflammation, whereas they can also recruit detrimental T-cells (not shown). On the other hand, CCR2− resident macrophages and macrophage subsets emerging at later time points following injury, assume a pro-reparatory phenotype by expressing and releasing pro-fibrotic and proangiogenic factors, mainly through activation of fibroblasts and ECM deposition, to promote cardiac repair, and also by recruiting pro-reparative neutrophils (PMN). In addition, macrophage phagocytosis action promotes cardiac repair and is probably prevented by cardiomyocyte signals. Moreover, fibroblasts interact with cardiomyocytes in a paracrine manner to promote hypertrophy and fibrosis and instruct macrophages to mediate inflammation by secreting agents such as GM-CSF. Chronic stress and injury: macrophage activation in early time points affects the outcome. The inflammatory macrophage expansion in early pressure overload injury, for instance, leads to fibrotic ECM and heart failure in later time points triggering detrimental T-cell expansion (not shown). Under chronic activation conditions (pressure overload, cardiomyopathies, aging) macrophages secrete factors promoting fibrosis (e.g., IL-10, osteopontin) and fibrosis mediated by activated fibroblasts (including myofibroblasts) stimulates additional pathways affecting cardiomyocyte integrity and function. This propagates the detrimental effects of inefficient regeneration and repair, leading to heart failure. Fibroblasts can also mediate inflammation by releasing inflammatory cytokines such as MCP1 and IL6.