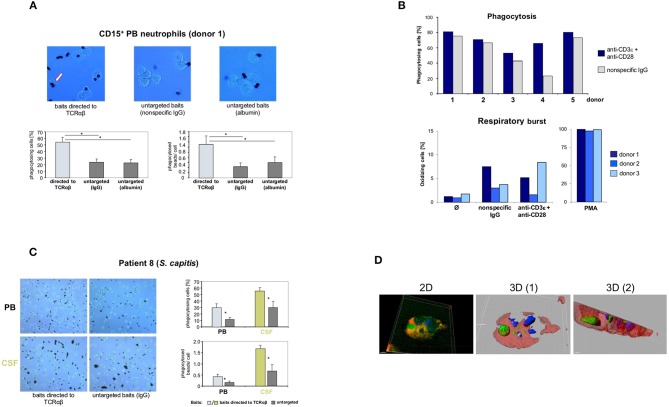
Figure 4.
Targeting of baits to the TCRαβ and TCRαβ activation via CD3/CD28 costimulation enhance neutrophil phagocytosis. (A) Representative unstained cytospin preparations (40x) of PB neutrophils from a healthy subject (donor 1) that were challenged with bead baits targeted to the TCRαβ or untargeted beads (controls) for 3 h. The arrow (left) highlights two phagocytosed beads. The quantitative analysis of neutrophil bead phagocytosis, defined as ingestion or binding of ≥1 bead, is shown in the bottom panel (percentage of phagocytosing cells, left; phagocytosed beads/cell, right). Note that targeting of beads to the TCRαβ significantly enhances PB neutrophil phagocytosis relative to untargeted beads. Purified PB CD15+ neutrophils were incubated with polystyrene bead baits (Ø 4.5 μm) coated with anti-TCRαβ antibodies and uptake of beads was recorded. Beads coated with equal amounts of non-specific IgG isotype antibodies, potentially binding to the Fcγ receptor, or albumin (irrelevant protein) served as controls (untargeted baits). See also Figures S18, S19 in the Supplementary material. (B) Anti-CD3/CD28-mediated TCRαβ activation enhances Fc receptor-independent phagocytosis of bacteria (top) but not respiratory burst in neutrophils (bottom). PB CD15+ neutrophils from five healthy individuals were infected for 10 min with non-opsonized FITC-labeled E. coli baits (cell/bacteria ratio 1:10) in the presence of anti-CD3ε/CD28 antibodies or non-specific IgG (top). Shown are the percentages of phagocytosing neutrophils. The bottom panel shows that CD3/CD28 costimulation under non-opsonizing conditions (three donors) has no effect on the neutrophil respiratory burst. Phagocytic and respiratory burst activities were assessed by flow cytometry. PMA, phorbol myristate acetate. (C) Increased phagocytosis of baits targeted to the TCRαβ in PB neutrophils and CSF neutrophils from a patient with Staphylococcus capitis meningitis (patient 8). PB and CSF neutrophils were simultaneously collected from the patient during the early phase of meningitis and subsequently challenged with beads targeted to the TCRαβ or untargeted beads (non-specific IgG) for 1.5 h. Representative cytospin preparations (20x, left) and the quantitative analysis of bead phagocytosis are shown (right). (D) 3D confocal immunofluorescence imaging identifies a CSF neutrophil from the same patient that has ingested a bead bait (green) targeted to the TCR. Two distinct cross-sections are shown (center, right). The 2D view is shown left. For a full 3D view see Movie S1. The bead bait was stained with FITC-labeled antibodies to mouse IgG, the cell membrane (red) and the nucleus (blue) were stained with Alexa 633-conjugated WGA and DAPI, respectively. All peripheral blood or CSF neutrophils were CD15+-MACS purified and incubated with bead baits at a cell density of 3 × 106/ml and a cell/bead ratio of 1:1. Quantitation of phagocytosed beads was conducted by bright field microscopy of at least 12 randomly selected fields of vision. Error bars represent mean ± SD. *p < 0.001, Dunnett post-hoc test (A), Student's pair test (C).