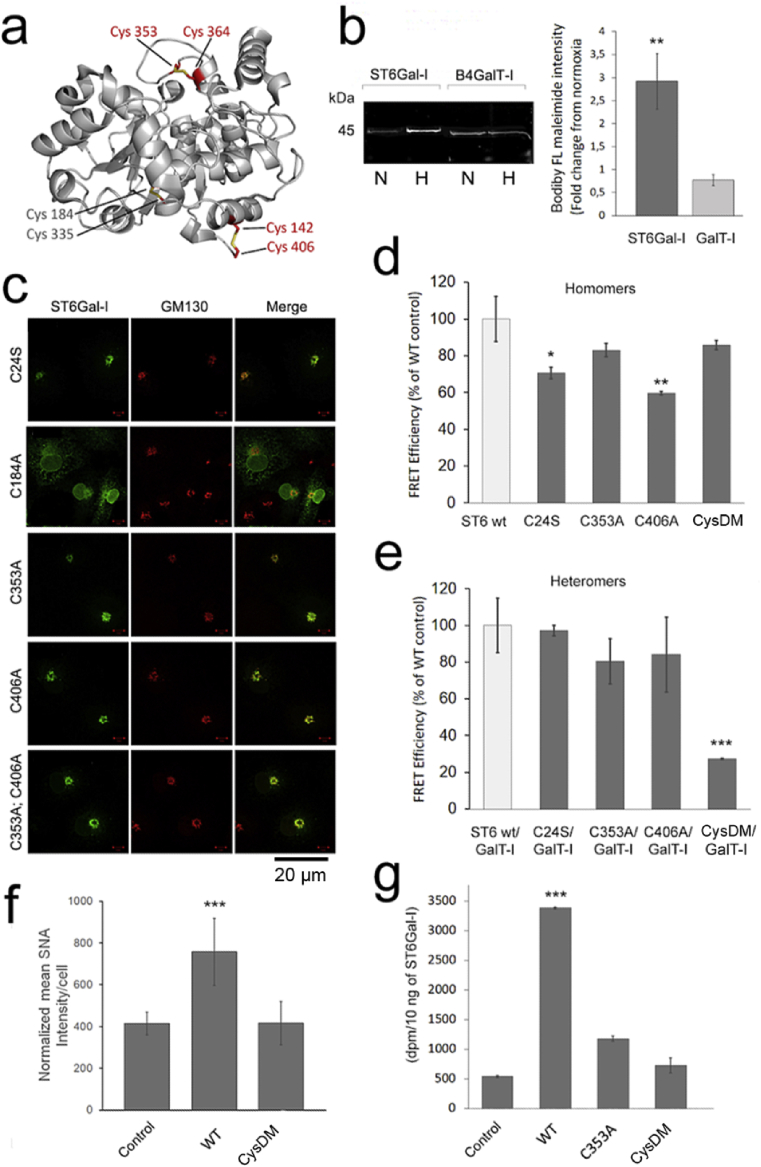
Fig. 5.
The effect of hypoxia on the formation of surface exposed disulfide bonds in ST6Gal-I catalytic domain. a) The crystal structure of the human ST6Gal-I catalytic domain (PDB code 4JS2) showing the 3 disulfide bonds between the depicted cysteine residues. Red text refers to surface exposed disulfide bonds while that grey text depicts an internal disulfide bond. b) Bodipy FL-maleimide staining of both ST6Gal-I and B4GalT-I proteins after growing cells for 20 h in normoxic or hypoxic conditions. The figure shows a representative BN-PAGE gel result after labeling of the cells and immunoprecipitation ST6Gal-I and B4GalT-I enzyme proteins with anti-HA tag antibodies from cell lysates prepared from normoxic (N) or hypoxic (H) cells). Band intensities were quantified from digitalized gel images using the ImageJ software. The bars (right) represent fold changes from the normoxic values (set to 1). Statistically significant changes relative to normoxic controls are marked with stars (p < 0.05*, p < 0.01**, p < 0.001***). c) Subcellular distribution of the various ST6Gal-I cysteine mutants. Cells were transfected with the indicated mVenus-tagged enzyme constructs, fixed and co-stained with the anti-GM130 antibody (Golgi marker) before imaging with the fluorescence microscopy. All constructs except the C184A colocalized well with the Golgi marker GM130. d-e) The effect of cysteine mutations on the assembly of the homomeric (d) and heteromeric (e) ST6Gal-I and B4GalT-I complexes. Cell treatments and quantification of the FRET efficiencies were done as above (for details, see the “Material and Methods” section). The bars denote to mean FRET efficiencies (% ± SD, n = 3, 15,000 cells each) from the wild type control values (set to 100%). Only statistically significant changes are shown and marked with stars (p < 0.05*, p < 0.01**, p < 0.001***). f) Quantification of the α-2,6-linked sialic acid levels by SNA lectin staining in control cells and cells expressing the ST6Gal-IWT or the ST6Gal-ICysDM mutant (C353, C406). In brief, 24 h post-transfection cells stained with the Alexa 488-conjugated SNA lectin and with the Hoechst dye to identify cells before quantification of the bound SNA lectin with the Operetta high content imaging system. The data are expressed as the mean SNA intensity/cell (±SD, n = 3, 15,000 cells each) after normalizing the intensities against the ST6Gal-I protein present in the cell lysates (EV Fig. 6). Statistically significant changes relative to control cells (mock-transfected cells) are marked with stars (p < 0.05*, p < 0.01**, p < 0.001***). g) Enzymatic activity of the ST6Gal mutants. Cells transfected with the indicated ST6Gal-I variants were lysed in RIPA buffer 24 h post-transfection, after which their activities were determined as described in the “Materials and Methods” section using asialofetuin as an acceptor. The values shown (bars) are expressed as the mean dpm/10 ng ST6Gal-I protein (n = 3) after normalizing them against the ST6Gal-I protein present (determined by immunoblotting as above). Statistically significant changes relative to control cells (mock-transfected cells) are marked with stars (p < 0.05*, p < 0.01**, p < 0.001***).