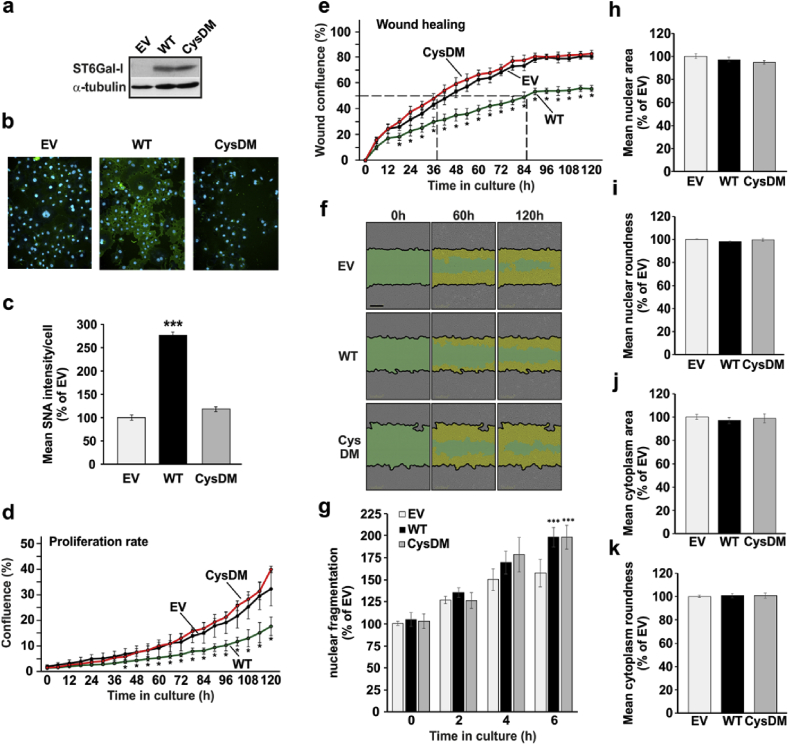
Fig. 7.
Functional consequences of the inactivation of the ST6Gal-I. a) Immunoblotting of the ST6Gal and its ST6Gal-ICysDM variant in COS-7 cells. Cell transfections were performed by electroporation before SDS-PAGE and immunoblotting with ST6Gal-specific antibodies (anti-CD75) as above. b) Representative figures on SNA lectin binding to the cells transfected separately with the same plasmids. Transfections and lectin staining was carried out as describe above. c) Quantification of the SNA lectin binding to cells using the high content imaging system. The data are shown as relative changes (mean % + SD, n = 3, 70–90000 cells/assay). d) Live cell proliferation rate analysis of COS-7 cells transfected with an empty vector (EV) or with vectors expressing the wild-type ST6Gal-I (WT) or the cysteine double mutant of ST6Gal-I (CysDM). Data are expressed as mean confluence % (±SD (n = 5) using a build-in Incyte™ protocol. The star (*) mark the statistically significant difference (p < 0.05) between relative confluence values of the wild-type ST6Gal-I (WT) and the cysteine double mutant (ST6Gal-ICysDM) at each time point. e) Live cell wound closure analysis of wild type (WT) and double cysteine (CysDM) ST6Gal-I variant expressing cells. The graph shows thee mean confluence (% ±SD (n = 4) at the indicated times after making the wound. Significant changes between confluence values between the wild-type ST6Gal-I (WT) and cysteine double mutant of ST6Gal-I (CysDM) at each time point are marked by stars (p < 0.05*). f) Representative images of the wound closure in COS-7 cells transfected with an empty vector (EV), ST6Gal-I (WT) or the cysteine double mutant of ST6Gal-I (CysDM) at 0 h, 60 h and 120 h time point after introduction of the wound. Scale bar 300 μm. Green color represents the original wound, and yellow color depicts the area of repopulated cells. g) Sensitivity cells to staurosporine-induced apoptosis. Cells transfected with the indicated ST6Gal-I variants were subjected to 5 μM staurosporine for 0–6 h. After fixing, cells were stained with the Hoechst dye before imaging high content imaging system (Operetta, PerkinElmer). Fragmentation index of nuclei was then calculated after image segmentation using the “RMS Nuclear Fragmentation” built-in protocol, in which the coefficient of variance percentage (CV%) of the nuclear intensity is used to calculate the fragmentation index. Healthy nuclei have low CV values whereas fragmented nuclei have higher CV values. The values represent the mean (±SD, n = 3, 15,000 cells each. Statistically significant changes are marked with stars (p < 0.05*, p < 0.01**, p < 0.001***). h-k) The effect of the wild type ST6Gal-I and its double cysteine variant on nuclear and cell morphology. Cells transfected with the indicated constructs were plated on a 96 well plate, fixed immuno-stained with an α-tubulin antibody, and counterstained with the Hoechst dye. After imaging (Operetta, PerkinElmer) and segmentation, in-built “Harmony” protocols were used to calculate nuclear and cytoplasmic areas (h and j) and their roundness (i and k). The data are expressed as the mean % (±SD, n = 3, 15,000 cells each) of the control values (EV = empty vector). No statistically significant changes were detected between the different ST6Gal-I transfectants (p > 0.05).