Figure 5.
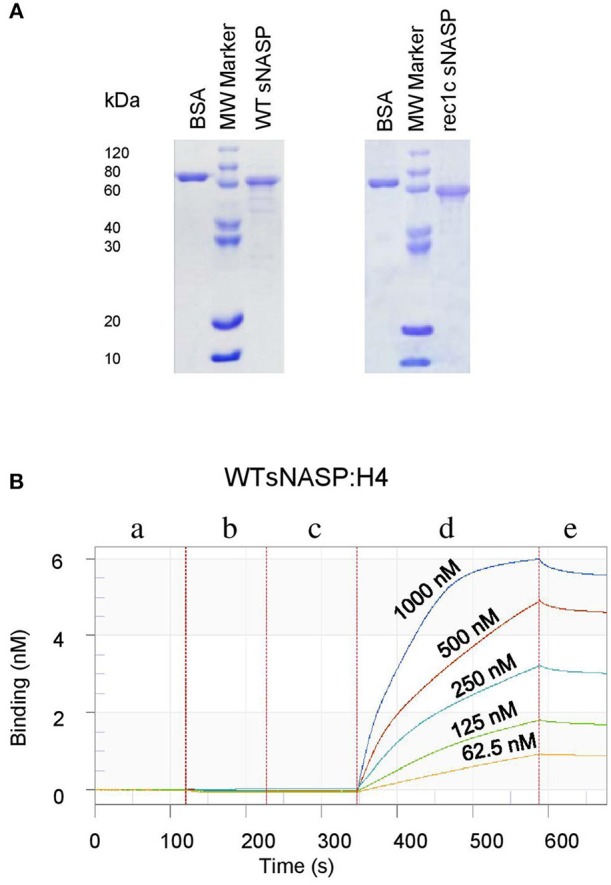
Expression, purification, and detection of histone-binding affinity of the mouse sNASP protein. Proteins were expressed in E. coli and purified using ion-exchange chromatography and size exclusion chromatography. (A) The purity of recombinant WT sNASP and rec1c sNASP proteins was more than 90% as confirmed by SDS-PAGE gel. Biolayer interferometer was used to detect the affinity of WT sNASP and rec1c sNASP proteins binding mouse histones H1a, H3.1, H4, and H3.1/H4 tetramer. (B) A graph of a representative assay shows the interaction of WT sNASP binding histone 4 at the indicated concentrations from 62.5 to 1,000 nM. Each experiment is represented by an initial baseline (a), a sNASP immobilization curve (b), another baseline (c), an association curve (d), and a disassociation curve (e).
