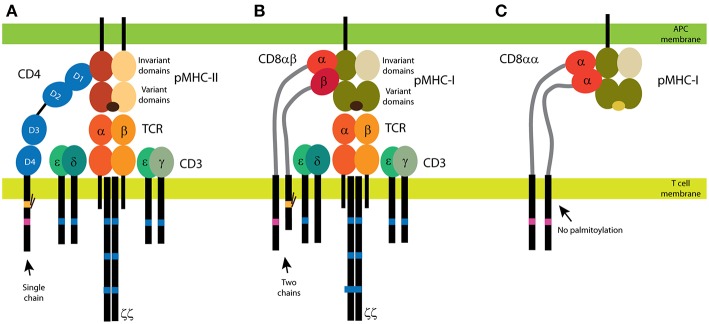
Figure 1.
CD4 and CD8 coreceptors. (A) The CD4 glycoprotein is composed of a single chain. Its functional motifs, such as the Lck-binding site (in magenta) and the palmitoylation site (in yellow), are in the sole intracellular domain. The extracellular part of CD4 is composed of four Ig-like domains, and the MHC binding site is in the N-terminal D1 domain. Short linker connects CD4 extracellular domains with the transmembrane domain. (B,C) Two forms of CD8 exist: the αβ heterodimer (B) and the αα homodimer (C). The α subunit of CD8 contains the Lck-binding site, and the β subunit contains the palmitoylation site. A single Ig-like domain and a long stalk region (in light gray) form the extracellular parts of the CD8 subunits. Binding of CD4 (A) and CD8αβ (B) to MHC is illustrated with the antigenic receptor because these coreceptors support receptor function in T cells. The TCR/CD3 complex is composed of at least eight subunits. CD3 subunits γ, δ, and ε contain one immunoreceptor tyrosine-based activation motif (ITAM; in dark blue) and three ITAMs are in each ζ subunit. Cognate peptides are depicted in dark brown, self-antigens in light brown.