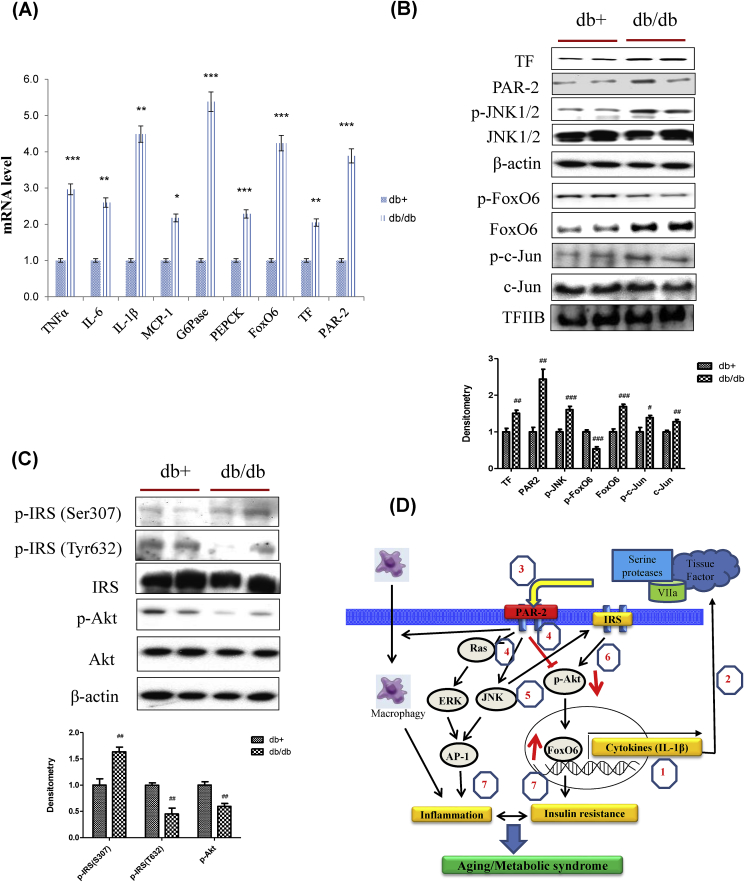
Fig. 8.
Obesity-induced inflammation and insulin resistance through TF/PAR2 signaling. (A) TNFα, IL-6, IL-1β, MCP-1, G6Pase, PEPCK, FoxO6, TF, and PAR2 mRNA. Real-time PCR analyses were performed to determine mRNA levels in liver tissues of db/db mice (n = 4 in each group). The results shown are representative of three experiments. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 vs. db+. (B) TF, PAR2, p-JNK1/2, JNK1/2, p-FoxO6, FoxO6, p-c-Jun, and c-Jun levels were assessed using nuclear and cytosolic proteins from db/db mice. TFIIB was the loading control of the nuclear fraction. β-actin was the loading control of the cytosolic fractions. Results are representative of three independent experiments. Bars in densitometry data represent means ± SE, and significance was determined using an unpaired t-test: #p < 0.05, ##p < 0.01, ###p < 0.001 vs. db+. (C) p-IRS (S307), p-IRS (T632), IRS, p-Akt, and total-Akt levels were assessed using cytosolic proteins from db/db mice. β-actin was the loading control of the cytosolic fractions. Results are representative of three independent experiments. Bars in densitometry data represent means ± SE, and significance was determined using an unpaired t-test: ##p < 0.01 vs. db+. (D) A possible mechanism underlying the effect of FoxO6 on PAR2 signaling in aging. PAR2, protease-activated receptor 2; FoxO6, Forkhead transcription factor O6. Figure out: 1, FoxO6 induced IL-1β; 2, secreted IL-1β mediated tissue factor; 3, Tissue factor activated PAR2; 4, PAR2 induced ERK and JNK, and inhibited Akt level; 5, JNK induced phosphorylation of Ser-IRS; 6, Phosphorylated IRS inhibited Akt; 7, Activated ERK and JNK induced inflammation by AP-1, dephosphorylation of Akt mediated insulin resistance through FoxO6 activation. However, activation of FoxO6 induced IL-1β again.