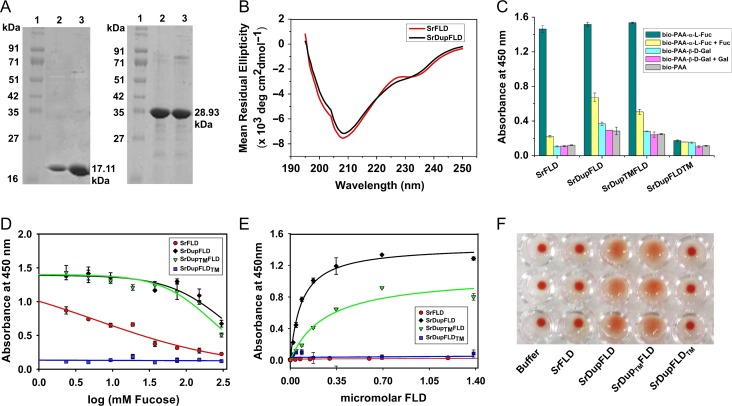
Fig. 2.
Glycan binding studies of SrFLD and SrDupFLD. (A) SDS-PAGE showing SrFLD (17.11 kDa) and SrDupFLD (28.93 kDa) purified using Ni-NTA metal ion affinity chromatography; Lane 1: Protein marker from Puregene; Lane 2 and 3: purified proteins. (B) Circular dichroism spectra of wild-type lectin, SrFLD and engineered lectin, SrDupFLD. (C) Histogram showing ELLA results for binding of SrFLD, SrDupFLD, SrDupTMFLD and SrDupFLDTM, to biotinylated PAA-α-l-Fucose and PAA-α-d-Galactose. (D) ELLA graph showing inhibition curves for l-fucose inhibition of SrFLD, SrDupFLD, SrDupTMFLD and SrDupFLDTM binding to biotinylated PAA-α-l-fucose. (E) Graph showing apparent binding of SrFLD, SrDupFLD, SrDupTMFLD and SrDupFLDTM to immobilized biotinylated PAA-α-l-fucose determined using ELLA. (F) Photographic image of agglutination of human type-O erythrocytes (in triplicate) by SrFLD, SrDupFLD, SrDupTMFLD and SrDupFLDTM. The buffer control is also shown.