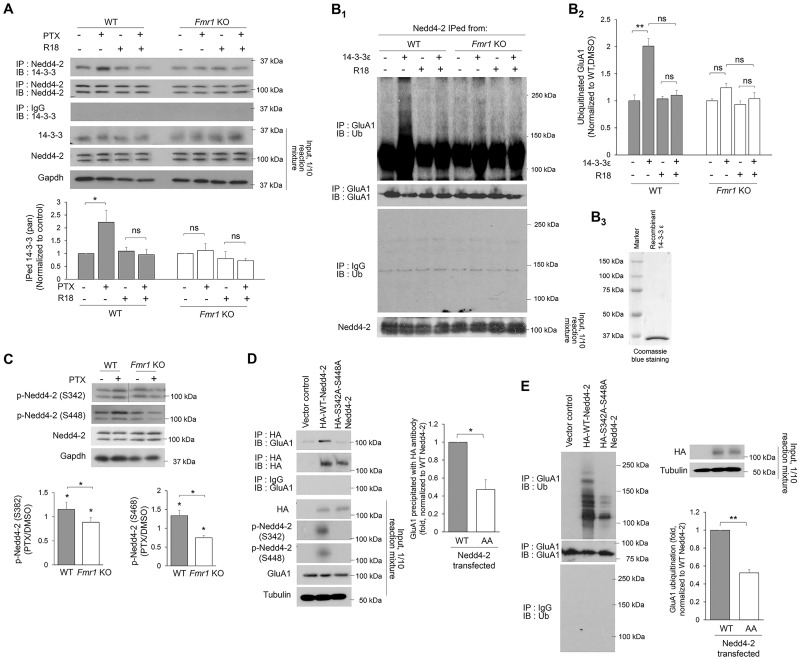
Figure 3.
Loss-of-function dephosphorylation of Nedd4–2 disrupts GluA1 ubiquitination in Fmr1 KO neurons. (A) Western blots of 14-3-3 after co-IP of Nedd4-2 using lysates from WT or Fmr1 KO cortical neuron cultures treated with vehicle (DMSO) or 14-3-3 inhibitor (R18) (0.025 mg/ml) for 1 h following vehicle (DMSO) or PTX treatment for 48 h (two-way ANOVA with Tukey’s test, n = 4). (B) Western blots of Ub or GluA1 after IP with anti-GluA1 antibody following in vitro ubiquitination with recombinant GluA1 and Nedd4-2 immunoprecipitated from WT or Fmr1 KO cortical neuron cultures treated with PTX for 48 h. An addition of recombinant 14-3-3ε or R18 was applied prior to the ubiquitination reaction as labeled in the figure (Two-way ANOVA with Tukey’s test, n = 4). Coomasie blue staining showing the purity of recombinant 14-3-3ε is on the right (B3). (C) Western blots of phospho-Nedd4-2-S342 and phospho-Nedd4-2-S448 from WT or Fmr1 KO cortical neuron cultures treated with DMSO or PTX for 48 h (one-sample t-test for each genotype and Student’s t-test for comparison between genotypes, n = 4). (D) Western blots of GluA1 after co-IP of HA-Nedd4-2 using lysates from HEK cells transfected with GluA1 and control vector, HA-WT-Nedd4-2 or HA-S342A-S448A-Nedd4-2 for 2 days. The intensity of precipitated GluA1 from all samples was normalized to HA-WT-Nedd4-2-transfected cultures (Student’s t-test, n = 4). (E) Western blots of Ub after IP using anti-GluA1 antibody and lysates from HEK cells transfected with GluA1 and control vector, HA-WT-Nedd4-2 or HA-S342A-S448A-Nedd4-2 for 2 days Student’s t-test, n = 3). Data are represented as mean ± SEM with *P < 0.05, **P < 0.01, ***P < 0.001 and ns: non-significant.