Abstract
Since the introduction of the first anti-tumor necrosis factor antibodies in the late 1990s, biologic therapy has revolutionized the medical treatment of patients with inflammatory bowel disease (IBD). Nevertheless, surgery continues to play a significant role in treating IBD patients. Rates of intestinal resection in patients with Crohn’s disease or colectomy in ulcerative colitis are reducing but not substantially over the long term. An increasing variety of biologic medications are now available to treat IBD patients in various clinical situations. Consequently, a number of questions persist about how biologic medications affect the need for surgery and overall course in IBD patients. Given the trend for earlier and more frequent use of biologic medications in IBD patients, a working knowledge of the effects of these medications on surgical decision-making and outcomes is essential for the practicing colorectal surgeon and gastroenterologist. This review seeks to summarize the relevant literature surrounding biologic use and IBD surgery with a focus on the effect of biologics on the frequency, type and complications of surgery in this ‘age of biologics’.
Keywords: Biologics, inflammatory bowel disease, surgery, review
Introduction
Inflammatory bowel disease (IBD), consisting of ulcerative colitis (UC), Crohn’s disease (CD) and indeterminate colitis (IC), is a broad group of autoimmune disorders primarily affecting the gastrointestinal tract. IBD has a prevalence of approximately 400 cases per 100,000 people in the USA and accounts for 3 to 6 billion dollars of health-care costs per year [1–3]. Surgery has historically been common in this population, with a lifetime risk of surgery ranging from 50% to 80% in CD patients and colectomy rate reaching 30% in UC patients [4–6]. In the past two decades, the introduction of the ‘biologics’ has revolutionized the medical approach to IBD, with use rates reported as high as 40% in CD patients and 16% in UC patients [7, 8]. However, despite the emerging trend for early and frequent biologic use for the management of severe IBD, biologic therapies have not eliminated or even significantly reduced the need for surgery in this patient population [5, 7, 9]. Thus, with an increased prevalence of surgical patients being treated with biologics, a working knowledge of the available biologic therapies and their effect on surgical care for the IBD patient is essential for the practicing colorectal surgeon.
What are the biologics?
Biologics are active compounds derived from living cells [10]. In the treatment of IBD, the term ‘biologics’ refers to monoclonal antibodies against inflammatory immune mediators. They are distinct from the immunomodulatory drugs discussed in the literature, which traditionally include medications such as azaiothioprine (AZA), 6-mercaptopurine (6-MP), methotrexate (MTX) and cyclosporine. There are currently three main classes of biologics: tumor necrosis factor (TNF)-α inhibitors (infliximab, adalimunab, centrolizumab pegol and golimumab), integrin α4β7 inhibitors (natalizumab and vedolizumab) and an inhibitor of cytokines IL-12 and IL-23 (ustekinumab). Additionally, there are biosimilars, which are biologic medicines that are highly similar to the original drug and are intended to have no meaningful differences in safety, purity and potency [11]. Biosimilars for infliximab and adalimunab are currently on the market in the USA. Biologics are administered intravenously or subcutaneously with dosing intervals ranging from weekly to every several months. Of note, occasionally, a new therapy for an inflammatory condition is referred to as a biologic. An example is the drug tofacitinib, an orally administered small-molecule Janus Kinase (JAK) Inhibitor recently approved for the treatment of UC and often discussed in the literature alongside the monoclonal antibodies.
What is the biologic era?
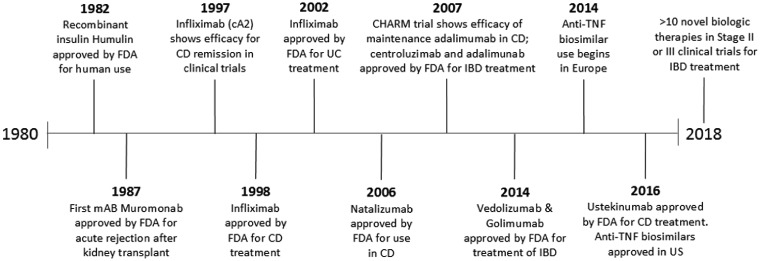
The first biologic approved for human use by the Food and Drug Administration (FDA) was a recombinant form of insulin called Humulin in 1982 [12]. The first monoclonal antibody drug was muromonab, an anti-T-cell antibody used to prevent rejection in kidney transplant. In 1997, the first randomized trials showed efficacy of the of monoclonal antibody cA2, which would later become infliximab, in the treatment of CD [13]. Infliximab was approved by the FDA for use in 1998. In 2007, both centroluzimab and adalimunab were approved for IBD in the USA. Five other monoclonal antibodies have followed in the subsequent decade, along with the first use of biosimilars for IBD in Europe in 2014 and in the USA in 2016 (Figure 1).
Figure 1.
Timeline of biologic agent introduction. FDA, Food and Drug Administration; mAB, monoclonal antibody; IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; TNF, tumor necrosis factor.
Since the FDA approval of infliximab, biologic use has expanded rapidly. In a 2005 study demonstrating the efficacy of centroluzimab in CD treatment, approximately 20% of participants already had prior exposure to another TNF-α inhibitor [14]. Population-based data from Western countries through 2011 indicate that 5%–10% of patients with IBD have been exposed to a biologic treatment; the proportion is even higher in referral centers, with as many as 30% of patients requiring surgery having been treated with biologics [9, 15–18]. More recently, a 2018 study of insured patients in the USA showed that use of biologics in CD patients had risen from 20% to 40% between 2009 and 2015, and from 5% to 16% in UC patients [19]. Given the earlier and more frequent use in severe IBD, as well as the ongoing emergence of new biologic therapies, it is apparent that we are now in the biologic era of treatment, and are likely to remain so for some time [8].
Crohn’s disease
Have biologics changed the rates of surgery in patients with CD?
The lifetime risk of bowel resection in CD patients has historically been very high. Some reports from the latter half of the twentieth century show the prevalence of intestinal resection in the range of 70%–80% [20, 21]. These percentages have improved over time and patients diagnosed in the 1990s had an approximate 14%, 28% and 39% risk of surgery at 1, 5 and 10 years, respectively, from initial diagnosis [22]. The first randomized trials of biologic therapy in luminal CD (ACCENT I and CHARM) took place in the early 2000s [23, 24]. The rates of surgical interventions in these study patients were impressively low, ranging from 0.6% to 3.0% at 1 year in the groups treated with regularly dosed biologic medication [25, 26].
However, these low rates of surgery in the initial randomized trials did not immediately translate into the general population. A number of observational studies show only a modest decrease, if any, in surgical rates, mostly evident within the first years of diagnosis [17, 27–32]. These studies and others have been comprehensively reviewed by several groups who generally agree that, in the years following initiation of the biologic therapy, 1- and 5-year surgical rates in CD patients range from 10%–20% to 20%–35%, respectively [6, 21, 22].
Predictably, the use of biologic therapy in these cohorts varied. In a study of 3400 patients from Manitoba, only 5% of patients after 2001 were being treated with infliximab and the 5-year risk for surgery in the total CD population after 2001 was 18% [17]. On the other hand, a report of 296 patients treated at Nancy University Hospital in France from 2000 to 2008 (all diagnosed after 2000) demonstrated 65% biologic usage overall [33]. Of all patients who would undergo surgery, 60% had been treated with at least one biologic agent. The majority of these patients received only infliximab, 26% received infliximab then adalimunab, 6% only adalimunab and 2% received infliximab then adalimunab and finally certolizumab prior to their operation. The Nancy cohort’s 5-year risk for surgery, however, was comparable to the Manitoba cohort at 25%.
Several observational studies have examined the rates of surgery in CD patients treated with a biologic (Table 1). Schnitzler et al. [34] found a 27.1% surgery rate among 614 patients treated at a single Belgian center with a median follow-up of 4.6 years. Subsequent evaluations of the Nancy cohort found that patients undergoing therapy with either infliximab or adalimunab had a cumulative 6.2% and 24.9% surgical rate at 1 and 5 years, respectively [31]. In a Dutch study of 469 consecutive CD patients treated with infliximab at two referral centers, the rates for abdominal surgery were 8.62/100 patient-years in the overall cohort and 6.06/100 patient-years in those receiving scheduled doses [35]. Median follow-up in this group was 4.5 years; importantly, however, primary non-responders were excluded. A single-center retrospective study in Canada demonstrated a markedly lower surgical rate, with only 5/71 (7%) with a median follow-up of 62 months [36]. There have been several other studies with shorter follow-up whose rates of surgery in biologic-treated patients range from 15% to 33% [37, 38, 40]. A single study examining surgical outcomes in patients treated with vedolizumab demonstrated a 9.2% surgical rate at 24 months [39].
Table 1.
Long-term surgical rates in patients with Crohn’s disease (CD) on biologic therapy
| Literature | Study period | Study design | No. of patients | Biologic used | Rate of surgery | Follow-up |
|---|---|---|---|---|---|---|
| Schnitzler et al. (2009) [34] | 1994–2007 | Retrospective single-center | 614 | Infliximab | 23.5% | 55 months (median) |
| Peyrin-Biroulet et al. (2016) [31] | 2000–2013 | Retrospective multi-center | 350 | Infliximab or adalimumab | 24.9% (calculated 5-year rate) | 33 months (median) |
| Eshuis et al. (2013) [35] | 1993–2010 | Retrospective multi-center | 276* | Infliximab | 18% no prior surgery for CD; 24% prior surgery for CD | 54 months (median) |
| Alzafir et al. (2011) [36] | 2002–2008 | Retrospective single-center | 71 | Infliximab | 7% | 62 months (median) |
| Caviglia et al. (2007) [37] | 1999–2005 | Retrospective single-center | 40 | Infliximab | 20% | 27 months (median) |
| Ljung et al. (2004) [38] | 1999–2001 | Population-based cohort | 191 | Infliximab | 17.2% | 24 months |
| Feagan et al. (2008) [39] | 2000–2002 | Randomized–controlled trial | 65 | Vedoliuzimab | 9.2% | 24 months |
Subgroup of patients who underwent maintenance therapy.
While it appears that biologics have reduced early surgical rates in CD patients, the low intestinal resection rates demonstrated in early clinical trials have not been borne out in practice [21]. Furthermore, the populations being treated with biologics continue to change. Many early studies examined patients with long-standing disease and a history of previous resection, whereas, now, with more aggressive endoscopic and radiologic surveillance and higher use of biologics in the community, patients inevitably encounter biologic therapy sooner in their disease process.
Given the evidence that current medical treatment has not obviated the need for intestinal surgery in CD patients, the interaction between these two therapeutic modalities must be further examined. The risks and benefits of initiating aggressive medical therapy on patients who will eventually require surgery merit exploration, as it remains unclear whether pre-operative biologic treatment may reduce the extent of surgical resection or instead increase patient perioperative risk without the benefit of preserving more bowel.
Do biologics prevent recurrent surgery in patients with CD?
Unlike UC, there is no surgical cure for CD; thus, repeat operations in CD patients are quite common, especially at sites of previous anastomoses. Rates of re-operation 5 and 10 years following an initial resection are close to 25% and 35%, respectively [41]. Similarly to overall operative rates, these have decreased from historical rates as high as 70%–90% [20]. Successive resections leading to progressive shortening of the small bowel can have devastating clinical consequences. However, recent studies have demonstrated the benefit of early resection, indicating that optimal treatment of CD (especially ileocecal disease) does not necessarily dictate minimizing resection [42, 43]. Thus, understanding whether biologics can affect recurrence and re-operation rates following an initial operation is an important factor in operative planning.
There have been a number of small trials comparing biologics to placebos as well as to immunomodulators in post-operative recurrence [44–47]. Endoscopic recurrence has been dramatically improved in the biologic group. Savarino et al. [45] demonstrated a 6% vs. 64% recurrence in endoscopic findings with adalimunab vs AZA at 2 years in 51 patients. Similarly, Yoshida et al. [46] saw 19% vs. 78% endoscopic recurrence at 1 year in 31 patients. Unfortunately, most of these trials had a small sample size and limited follow-up, and focused on endoscopic findings and clinical scores rather than repeat operations. The overall trend in these initial small studies, however, is that biologics appear superior to both placebos and immunomodulators in preventing post-operative CD recurrence.
Other studies have not shown a superiority of biologics in the post-operative period. Magro et al. [48] examined patients treated with AZA or AZA combined with infliximab and did not see a significant difference in the number of surgeries required. Recently published results of a blinded randomized–controlled trial (RCT) comparing post-operative adalimunab with AZA did not show any significant differences either in endoscopic recurrence or surgical rates [49]. In this patient population from Spain, the difference in 52-week re-operation rates between the two arms (4% and 7% in the adalimunab and AZA arms, respectively) was not statistically significant. Of note, patients did not receive adalimunab drug-level monitoring in this study, which has been shown to improve the efficacy of adalimunab treatment [50].
The PREVENT trial is a multi-center RCT testing whether a scheduled dosing regimen of infliximab prevents recurrence in ‘high-risk’ post-operative CD patients [51]. At a median follow-up of 84 weeks, the investigators saw a reduction in endoscopic recurrence but not in clinical endpoints. Interestingly, surgery rates were very low, at between 1% and 2% in both the placebo and infliximab groups. When interpreting results in recurrent CD, it is important to remember that endoscopic recurrence is predicative of ultimate clinical recurrence, and thus longer-term results from these cohorts will be of great interest [52].
The POCER RCT also investigated optimal post-operative medical care for CD patients by comparing active endoscopic surveillance and a ‘step-up’ methodology with empiric drug selection [53]. Results were better in the active endoscopic surveillance and management group. This group also followed patients initially treated with adalimunab in the post-operative period due to thiopurine intolerance. While results were not significant, there did seem to be a trend towards improved results with immediate post-operative adalimunab. Taken together, these studies suggest that there is a benefit to biologic therapy compared to placebo post-operatively in high-risk CD patients, although a relative benefit over thiopurines may not be as clear.
When is it safe to start a biologic after surgery?
There are limited data regarding the optimal timing of initiation of biologic therapy in the post-operative period. Some studies have been equivocal about the benefit of early initiation [54]. However, historical data tell us that 90% of patients will have evidence of recurrence within 1 year [55]. The American Gastroenterological Association guidelines recommend early pharmacologic prophylaxis within 8 weeks of surgery [56]. The trend in most trials with ‘high-risk’ patients is to initiate therapy within 4 weeks. Data from randomized studies have not demonstrated an increased risk of adverse events with biologics vs placebos or biologics vs thiopurines when initiating early therapy [49, 51, 53, 57]. When planning to start biologic therapy post-operatively, there does not seem to be any additional risk to initiating within 4 weeks.
The issue of withdrawing biologic therapy once in clinical remission is both complicated and outside the scope of this review, although, with the growing prevalence of biologic therapy, the subject has been discussed often in recent years [58–60]. There have been limited studies on withdrawing biologic therapy in post-operative CD patients, all with dismal results indicating greater than 50% recurrence rate, mostly within 18 months [44, 47, 61].
Do biologics increase perioperative complications in patients with CD?
There is an increased rate of perioperative complications in CD patients compared to the general surgical population [62–64]. Specific risk factors implicated in increased intra-abdominal complications include low serum albumin, pre-operative steroid use and pre-operative abscess [65, 66]. The effect of biologic therapy on perioperative complications is one of the most controversial topics in IBD surgery currently. The broad immunologic effects of biologic medications, as well as laboratory evidence demonstrating the role of TNF-α in wound and intestinal anastomosis healing, led to concerns about increased perioperative complication rates in patients on biologic therapy [67–69]. Over 20 retrospective studies well documented in a number of recent reviews have examined this question, and yet there is no clear answer, likely due to the varying results, heterogeneous populations and lack of precisely defined pre-operative biologic use (defined in most studies as any use within 3 months of surgery) in the retrospective data [70–74].
Acknowledging these limitations, there have been several meta-analyses published from which some conclusions can be drawn [75–80] (Table 2). These meta-analyses conclude that there is an increased risk of perioperative complications in patients on pre-operative biologic therapy. The most consistently elevated risk was infectious complications, which occurred in approximately 20% of patients (OR approximately 1.5 across four studies, compared to no pre-operative biologic therapy). There was disagreement regarding the effect of biologic use on anastomotic complications, as well as total complications, although most showed total complication rate in excess of 20% and as high as 56%. Additionally, patients treated with pre-operative infliximab were more likely to be on combination therapy with immunomodulators, including, but not limited to, corticosteroids.
Table 2.
Summary of meta-analyses of perioperative complications in patients with Crohn’s disease on biologic therapy
| Literature | Significant increase in anastomotic complications? | Significant increase in total infectious complications? |
|---|---|---|
| Narula et al. (2013) [75] | Not separately examined | Yes, RR = 1.93 (95% CI: 1.28–2.89) |
| El-Hussuna et al. (2013) [76] | Yes (low bias studies), RR = 1.63 (95% CI: 1.03–2.60) No (medium bias studies), RR = 0.17 (95% CI: 0.05–0.60) | No, RR = 1.15 (95% CI: 0.86–1.53) |
| Kopylov et al. (2012) [77] | No, OR =1.18 (95% CI: 0.61–2.30) | No, OR = 1.62 (95% CI: 0.92–2.86) |
| Billioud et al. (2013) [78] | Not separately examined | Yes, OR = 1.45 (95% CI: 1.03–2.05) |
| Waterland et al. (2016) [79] | No, OR = 1.19 (95% CI: 0.82–1.71) | Yes, OR = 1.52 (95% CI: 1.14–2.03) |
| Yang et al. (2014) [80] | Not separately examined | Yes, OR = 1.47 (95% CI: 1.08–1.99) |
OR, odds ratio; RR, relative risk; CI, confidence interval.
Do serum drug levels matter at the time of surgery?
As experience with biologic treatment grows, there is an understanding that treatment history and timing are insufficient to assess drug activity. Several studies have correlated serum drug levels with clinical outcomes [81–84] (Table 3). Lau et al. [82] reported a single-surgeon series of close to 200 IBD patients undergoing abdominal surgery over 13 years where serum samples had been drawn in the perioperative period. Several interesting findings came out of this study. The first is that only 53% of patients (75/142) who reported TNF-α inhibitor use had a detectable level at the time of operation. Patients with CD as compared to those with UC were more likely to have a detectable level (75% vs. 25%) and those levels were likely to be higher. The overall complication rate in this series was 31%, with a trend towards significance for increased risk of complications in those patients with a detectable TNF-α inhibitor level. However, only in the subgroup analysis of patients with a serum drug level above or below 3 μg/mL did these comparisons reach statistical significance. In this group, the risks of total complications were 34% and 17% and infectious complications were 23% and 9% for the high and low drug levels, respectively.
Table 3.
Biologic agent level and post-operative complications
| Literature | Study period | Study design | No. of patients | Serum biologic level | End point | Complications | Significant difference? |
|---|---|---|---|---|---|---|---|
| Waterman et al. (2013) [84] | 2000–2010 | Retrospective single-center | 19 UC | Detectable vs. Not (cutoff 1.4 μg/mL) | Overall infectious complications | 3/10 (detectable) vs. 0/9 (not detectable) | No |
| Lau et al. (2015) [82] | 1999–2012 | Retrospective single-surgeon | 60 UC | Detectable vs. Not | 30-day complication | 8/17 (detectable) vs. 17/43 (not) | No for Detectable vs. Not |
| Stratified: (>3 vs. <3 μg/mL)* | 16/47 (>3 μg/mL) vs. 13/76 (<3 μg/mL) | Yes (OR 2.5, P = 0.03) for >3 vs. <3 μg/mL | |||||
| 123 CD | |||||||
| Fumery et al. (2016) [83] | 2010–2014 | Multi-center prospective cohort | 76 CD | >1 and >3 μg/mL | 30-day complication | Only OR reported | No |
| >1 μg/mL: 0.69 (95% CI: 0.21–2.22) | |||||||
| >3 μg/mL: 0.95 (95% CI: 0.28–2.96) |
Subgroup analysis of low or undetectable (<3 μg/mL) vs. medium and high (>3 μg/mL). UC, ulcerative colitis; CD, Crohn’s disease; CI, confidence interval.
On the other hand, Fumery et al. [83] reported outcomes of a prospectively gathered database of adult CD patients undergoing ileocecal resections. In this cohort of 209 patients, 44% and 21% reported TNF-α inhibitor use within 3 and 1 months before surgery, respectively. Serum drug levels were measured in 76 patients and found to be >1 μg/mL in 40 patients and >3 μ/mL in 36 patients. The overall complication rate in the cohort was 20.5%. Neither TNF-α inhibitor use nor level was associated with increased risk of complications. Multivariate analysis demonstrated that only pre-operative steroid use within 1 month before surgery was significantly associated with increased risk of complications (OR 2.69, 95% confidence interval (CI): 1.15–6.29). Although reconciling the results of these contradictory studies is difficult, both studies highlight the variability of serum drug levels in perioperative IBD patients—notably that approximately half of patients with a history of recent biologic use had a serum level of less than 1 μg/mL at time of surgery.
Do non-TNF-α inhibitor biologics carry the same risk of complications?
There are limited data on non-TNF-α inhibitor biologics and perioperative outcomes but, predictably, the studies disagree on their effect. Two studies have examined perioperative outcomes with vedolizumab [85, 86]. Mechanistically, the gut specificity for vedolizumab held promise for fewer systemic side effects. Lightner et al. [85] conducted a retrospective single-center review of 94 IBD patients who had received vedolizumab within 12 weeks before major abdominal surgery, compared to contemporary controls treated with TNF-α inhibitors and non-biologic therapy. They found a significant increase in overall complications—53% for vedolizumab vs. 33% for TNF-α inhibitors, and 28% for non-biologics—of which most were surgical-site infections. Meanwhile, Yamada et al. [86] conducted a similar retrospective study, including all operations while limiting inclusions for drug exposure to within 4 weeks before the operation. Neither in total nor in the CD subgroup analysis was either vedolizumab or TNF-α inhibitor treatment associated with higher risk of complication infectious. The overall complication rate was 23.4% for vedolizumab vs. 31% for TNF-α inhibitors and 35.6% for non-biologics.
Given the contradictory results, heterogeneity in populations and evolving understanding and administration of biologic therapy, it is difficult to make generalizations about the effect of biologic medication on perioperative outcomes. Nevertheless, the data can support several conclusions. First, the rate of perioperative complications in CD patients remains high in the era of biologics and is likely modestly increased in those who have taken biologics. Whether this is a causal association or simply correlation between biologic use and disease severity—as well as multimodal treatment further increasing complication risk—remains unclear. Additionally, a history of a recent dose of biologic medication can confer very different effects on individual patients and measurement of biologic level may become increasingly important in pre-operative workup. Finally, with the approval and adoption of other classes of biologic drugs and the inevitable entry of biosimilars into the market, the only sure thing is that some element of uncertainty will persist.
Have biologics changed surgery in perianal CD?
Perianal CD, characterized mostly by fistulae, abscesses, strictures and ulcers with associated pain and loss of continence, likely affects around 40% of CD patients over their lifetime [87, 88]. Prior to the biologic era, treatment focused around surgical drainage, antibiotics and immunomodulators. Depending on the study, between 5% and 20% of all CD patients required surgical intervention for perianal involvement and half to two-thirds of patients with perianal disease require intervention [89–91]. In the pre-biologic era, there was a 10%–20% rate of proctectomy in complicated perianal disease and fecal diversion rates were high [89, 92, 93].
Early trials of infliximab demonstrated improvements in both partial and complete healing of perianal fistula, as well as increasing the time to relapse in the infliximab-treated group compared to placebo [94, 95]. However, symptom resolution occurred in only 46% of initially treated patients and fistulas recurred in 22% of biologic-treated patients at 52 weeks. Subsequent studies also demonstrated the benefit of adalimunab but, again, complete healing rates were low, at only approximately one in three patients [96]. A recent review of outcomes in biologic therapy summarizes some of the reporting inconsistencies in studies to date [97]. While some studies demonstrate a benefit in the majority of patients, complete healing or clinical absence of symptoms occurs in only approximately 30%–40% of patients with biologic treatment.
There is an increasing body of data advocating a combined approach of exam under anesthesia, surgical drainage of abscess if necessary, non-cutting seton placement and biologic therapy initiation as the initial therapy for perianal CD [98–102]. Multiple single-center cohorts have reported their experience with this approach, although no randomized trials have been conducted. El-Gazzaz et al. [99] reported 218 patients with a mean follow-up of 3 years who had undergone surgery for perianal CD. Improvement was seen in 71.3% in patients on biologics but only 35.9% in patients without. Complete healing was only seen in 36.6% of patients in biologics, which was increased but not significantly from 26.5% patients not on biologics. Seton placement followed by fistulotomy and finally endorectal advancement flap were the most common operations.
Gaertner et al. [100] presented a retrospective series of 226 patients with high rates of success in both the infliximab-treated and untreated group (60% vs. 59%); however, the infliximab-treated group healed faster, in an average of 6 vs. 12 months (mean follow-up 30 months). Additionally, the most common procedure in the infliximab group was seton drainage (62%), whereas, in the non-infliximab group, fistulotomy occurred in 50%. Active proctitis was more than twice as prevalent in the infliximab group as well (90% vs. 40%). Rates of proctectomy (8% vs. 10%) and fecal diversion (6% vs. 6%) were comparable between the subgroups. Haennig et al. [101] presented 81 patients of whom 80% were treated with drainage if necessary and seton placement followed by infliximab infusion. With a median follow-up of 64 months, 87% of all patients initially healed. However, 44% recurred and 67% of those patients healed again. Shorter duration of seton drainage <2.5 months and shorter duration of infliximab <2.5 months were associated with healing. Of note, 90% of the patients in this cohort were on combination therapy with either AZA or MTX. Bouguen et al. [102] reported the results from the Nancy cohort of perianal CD treated with infliximab. In this cohort of patients, 63% underwent seton drainage and 64% healed at least one fistula within this cohort, although there was a 33% recurrence. In their multivariate analysis, they identified combination therapy of infliximab with immunosuppressant medication, as well seton removal prior to 34 weeks, as two distinct positive predictors of fistula closure.
For complex intrasphincteric or transphincteric fistulae that are not amenable to fistulotomy, there are a number of procedures including fibrin glue, porcine plug, advancement flap and ligation of the intersphincteric fistula tract, all of which have been performed in the CD population mostly in small studies. The results are mixed and have been summarized well in other reviews; the effect of biologic therapy on these treatments has not been studied [103, 104]. Local injections of biologics have been suggested and two small open-label trials have been performed with adalimunab and infliximab [105, 106]. Early results were promising but each trial had fewer than 20 patients and no larger or randomized trial has yet been published.
Population-based data from England do show that the rate of proctectomy has decreased in the biologic era. Proctectomy made up 16% and 14% of perianal surgeries in CD patients from 1989–1995 and 1996–2001, respectively. However, proctectomy comprised only 6% of perianal CD surgeries from 2002 to 2009 [89]. One meta-analysis did examine studies of fecal diversion for the treatment of perianal CD before and during the biologic era [107]. Overall, 64% of patients improved with fecal diversion and 41% of patients eventually underwent proctectomy. They found that, during the biologic era, restoration was more often attempted (44% vs. 31%, P = 0.18) but there was no significant difference in durable success of restoration (18% vs. 14%).
Similarly to data surrounding perioperative complications and the biologic drugs, the data surrounding optimal treatment for perianal CD in the biologic age are both heterogeneous and mostly retrospective. A recent systematic review examining the effect of biologics on combined medical–surgical therapy of perianal CD concluded that the studies were too heterogeneous to perform meta-analysis statistics [98]. That being said, those authors suggested (as do we) that a combined approach with surgical drainage, non-cutting seton placement and biologic therapy with or without additional immunomodulators is likely the best treatment in the biologic age. Again, significant questions remain. Is combination therapy with immunomodulators better than biologics? How will biologic treatment affect seton management? Will it meaningfully alter the success of fistula operations, and thus the risks and benefits of possible sphincter-damaging procedures? Currently, the PISA RCT is being run in Europe to address some of these questions; however, no results have been published yet [108].
Ulcerative colitis
Have biologics reduced the need for colectomy in patients with UC?
Between 10% and 30% of UC patients will ultimately require a colectomy [4, 22, 109]. Patients undergo total abdominal colectomy, proctocolectomy or, in rare cases, partial colectomy for the management of dysplasia or cancer, medication intolerance or, in the vast majority (∼90%) of cases, for medically refractory disease [110]. Biologic therapy for UC was first approved in the mid-2000s. Early trials showed that biologics were effective in staving off urgent or emergent colectomy in the setting of active disease [111–113]. However, many patients continue to ultimately require colectomy, generating questions about how biologic use should affect surgical decision-making in terms of timing, approach and choice of operation [5, 114]. Patients with UC present to surgeons in various states of disease severity, from fulminant colitis requiring emergent operation to asymptomatic dysplasia found on colonoscopy, in the setting of well-controlled disease. Such a wide variety of presentations in this group of patients makes generalized recommendations about their perioperative treatment challenging, if not impractical [114].
There have been several randomized–controlled studies examining colectomy rate following treatment with infliximab versus placebo. The largest of these studies were the ACT I/II studies in which 728 patients with moderately to severely active UC were randomized to treatment with infliximab or placebo [111, 112]. At 54-week follow-up, the colectomy rate was 10% in the infliximab-treated group and 17% in the placebo group. Importantly, patients who had received steroids within 2 weeks or were deemed likely to require colectomy were excluded. A smaller RCT examined 45 patients with moderately to severely active colitis following a 3-day treatment with IV steroids, who were then randomized to receive infliximab or placebo [113]. At 3-month follow-up, 29% of infliximab patients and 67% of placebo patients had required colectomy; however, at 3-year follow-up, 50% of patients from the infliximab treatment group had undergone colectomy [115]. Further information about long-term colectomy rates can be seen in two studies comparing infliximab and cyclosporine for steroid-resistant UC flares [116, 117]. The CONSTRUCT trial demonstrated a 41% colectomy rate at 3 years in patients initially treated with infliximab [116]. Meanwhile, Laharie et al. [118] demonstrated a 17% and 21% colectomy rate at 3 months for cyclosporine- and infliximab-treated patients, respectively. Follow-up data for the infliximab group at 1 and 5 years showed rates of colectomy-free survival to be 69% and 65%, respectively.
There have been a number of retrospective studies reporting colectomy rates in biologic-treated UC patients, with notable heterogeneity in study populations [118–127] (Table 4). Patients on a spectrum of disease from severe acute colitis to chronic refractory colitis were followed, and the number of patients continued on biologic maintenance therapy varied between studies. Overall, the colectomy rate ranged from 14% to 53%. In two of the largest cohorts—the Leuven (121 patients) and Nancy (191 patients) cohorts—colectomy rates were comparable at 17% (median follow-up of 33 months) and 18.8% (median follow-up of 18 months) [120, 121]. More recently, Swedish investigators reported their experiences with both acute refractory colitis and chronic active colitis. The acute refractory colitis cohort of 211 patients showed a 71% rate of colectomy-free survival at 3 months, which fell to 64%, 59% and 51% at 1, 3 and 5 years, respectively [122]. Meanwhile, the chronic active colitis group had an overall colectomy rate of 27% at 2.9 years [123]. Finally, Baki et al. [125] presented an interesting cohort treated as outpatients in a ‘real-world’ setting. They demonstrated a 20% colectomy rate during a median follow-up of 27 months following initiation of biologic therapy. Among those patients, 28% were treated with both infliximab and adalimunab; of note, the median duration of biologic therapy prior to colectomy was 9 months (range 2–44 months) and patients with acute severe colitis were excluded.
Table 4.
Long-term surgical rates in patients with ulcerative colitis (UC) on biologic therapy
| Literature | Study period | Study design | No. of patients | UC phenotype | Biologic used | Rate of colectomy |
|---|---|---|---|---|---|---|
| Gustavsson et al. (2010) [115] | 2001–2004 | Randomized– controlled trial | 24 | Acute steroid refractory (hospitalized) | Infliximab | 29% (7/24) at 3 months |
| 50% (12/24) at 3 years | ||||||
| Williams et al. (2016) [116] | 2010–2013 | Randomized– controlled trial | 135 | Acute steroid refractory (hospitalized) | Infliximab | 28% (38/135) at 3 months |
| 41% (55/135) at 3 years | ||||||
| Laharie et al. (2017) [117, 118] | 2007–2015 | Randomized– controlled trial | 57 | Acute steroid refractory (hospitalized) | Infliximab (initial) | 17% (10/56) at 3 months |
| 36% (20/56) during a median follow-up of 5.4 years | ||||||
| Mortensen et al. (2011) [127] | 1999–2008 | Retrospective multi-center | 56 | Acute steroid refractory (hospitalized) | Infliximab | 30% (17/56) at 3 months |
| 39% (22/56) during a median follow-up of 2.6 years | ||||||
| Ferrante et al. (2008) [120] | 1999–2005 | Retrospective single-center | 121 | Refractory UC (outpatient infusions) | Infliximab | 17% (21/121) |
| Oussalah et al. (2010) [121] | 2000–2009 | Retrospective multi-center | 191 | Mixed presentation of UC (2/3 with active colitis) | Infliximab | 19% (36/191) during a median follow-up of 1.5 years |
| Sjöberg et al. (2011) [122] | 1999–2010 | Retrospective multi-center | 211 | Moderate–severe steroid refractory UC (hospitalized) | Infliximab | 29% (62/211) at 3 months |
| 41% at 3 years | ||||||
| 47% at 5 years | ||||||
| Angelison et al. (2016) [123] | 2004–2011 | Retrospective multi-center | 250 | Chronic active UC (outpatient) | Infliximab | 14% (27/190) during a median follow-up of 2.9 years |
| Baki et al. (2015) [125] | 2011–2014 | Retrospective single-center | 72 | Ambulatory UC patients | Infliximab or adalimumab | 21% (15/72) during a median follow-up of 2.25 years |
Several trends emerge from examination of these studies. First, several studies highlight the markedly high rate of colectomy (∼50%) in initial non-responders to biologic therapy. Additionally, the vast majority of colectomies occur in the first 2 years following initiation of treatment in these cohorts. Finally, smaller studies with earlier publication dates appear to have higher colectomy rates. Ultimately, a 2013 systematic review and meta-analysis of this literature presented mixed results regarding the effect of infliximab treatment on colectomy [128]. As expected, RCTs showed a reduced risk of major abdominal surgery in infliximab-treated patients with a numbers needed to treat of 11 for 1.2 years; meanwhile, pooled results from observational studies showed a non-significant increase, although authors urge skepticism in the interpretation of this finding given the heterogeneity of study populations and lack of adjustment for disease severity.
Several population-based studies have compared colectomy rates just prior to the introduction of biologics and after their adoption [15, 18, 129, 130]. Jeuring et al. [15] broke down a Dutch population of UC patients into three cohorts from 1992–1997, 1998–2005 and 2006–2010 and examined early (<90 days from diagnosis) and late (>90 days from diagnosis) colectomy rates. Early colectomy rates decreased from 1.5% in the 1992–1997 cohort to 0.5% in both the 1998–2005 and 2006–2010 cohorts. Late colectomy rates showed no clear trajectory at 4.0%, 5.2% and 3.6%, respectively. Biologic use was 4.4% and 10.6% in the latter two cohorts. Rungoe et al. [18] used a nationwide Danish registry and compared cohorts from 1995–2002 and 2003–2011, with similar findings: the 1-, 4- and 9-year colectomy rates were all mildly decreased in the 2003–2011 cohort, from 4.7% to 4.0%, 8.4% to 7.5% and 10.4% to 9.1%, respectively. Biologic use was 2% and 9%. Similarly, a study from Edmonton, Canada, showed that the colectomy rate had been increasing prior to 2005 and then began to decrease, with a steeper reduction in emergent compared to elective colectomies [129]. A 2013 meta-analysis showed decreasing 1-, 5- and 10-year colectomy rates (4.1%/9.9%/13.7% vs. 2.7%/7.6%/Not reported) from incident cases between 1990–2011 and 2000–2011 [22]. In contrast, however, a study of a large US insurance database comparing patients diagnosed with UC in 2003 and 2011 found an increasing rate of colectomy at 2 years from 1.9% to 3% [130].
Some studies have also examined risk factors for colectomy in these populations [16, 109, 122, 131–133]. As expected, the extent of colitis and flare duration are associated with increased risk of colectomy. Additionally, several studies show that biologic use in itself is a risk factor for colectomy [109, 133]. Male sex and increasing age are associated with colectomy in some studies but not others, and several studies highlight that those from rural or non-university-associated geographical locations have a higher risk for colectomy [15, 133]. Unsurprisingly, hospitalization for a flare also predicts colectomy [132, 133].
Despite the heterogeneity of studies and in some cases contradictory results, there are several conclusions that can reasonably be made from the present literature about colectomies in UC. First, biologics can serve to delay colectomy in many patients with moderate to severe colitis undergoing a flare. However, while overall colectomy rates have decreased in recent decades, it is not clear that biologic treatment has substantially changed long-term colectomy risk. Being treated with a biologic indicates a more severe phenotype and some of these patients will ultimately require colectomy regardless of treatment strategy. Further study is necessary to help predict which patients will progress to colectomy, with better accuracy than can currently be predicted based on anatomical extent and temporal duration of disease.
Do biologics change the type of operation in patients with UC?
Total proctocolectomy followed by ileal pouch reconstruction is the gold standard for surgical treatment of UC. This is commonly performed in two or three steps and rarely in a single operation [134]. Multiple factors including pre-operative patient health and nutritional status, elective vs emergent setting and surgeon preference influence the choice of which operations are offered to UC patients seeking surgical cure [135]. Given concern about perioperative complications negatively affecting long-term pouch function, uncertainty about the perioperative effects of biologic medications and the influence that biologic medications have had on the overall health of patients seeking cure for medically refractory UC, it is no surprise that there is much debate about the choice between two- and three-stage operations in the biologic era.
There has been heterogeneity in the single-center studies that reported surgical experiences with UC patients on biologic treatment [136]. Owing to initial concern over the increased rate of perioperative complications, some argued that a three-stage approach was preferred in UC patients with recent biologic use [137, 138]. That being said, other centers have demonstrated that approximately 80% of ileal pouch-anal anastomosis (IPAA) operations can be done in two stages, even with increasing percentages of biologic-treated patients, and have reported equivalent, if not superior, outcomes with a two-stage approach [139, 140].
Several population-based studies have demonstrated changing trends in two-stage vs three-stage surgeries in the years since biologic therapy began [9, 141–144]. Two separate analyses of the Nationwide Inpatient Sample reveal several trends in the operative approach and patient characteristics since the introduction of biologics [9, 141]. The first is that the rate of total abdominal colectomy without proctocolectomy or pouch reconstruction as the first operation for UC patients is on the rise from ∼40% in the 1990s to 55% in 2011, with 2008 being the year that total abdominal colectomy became the most common procedure. The authors also reported an increase in malnutrition rates from 9.3% in 2003 to 15.6% in 2012, and a decrease in non-elective surgery from 38% to 27%. These numbers hint at the presence of a subpopulation of UC patients within the biologic era who, rather than receiving emergent operations, instead undergo prolonged medical therapy while chronically ill, developing significant malnourishment that precludes them from undergoing pouch reconstruction at the time of initial surgery.
Notably, these results are somewhat incongruent with an analysis of the NSQIP database from 2005 to 2011 [143]. Here, only patients with chronic UC were examined and emergent cases were excluded. In this population, two-stage operations increased in popularity throughout the biologic era, becoming more common than three-stage in 2006 and ultimately comprising just under 80% of non-emergent operations for chronic UC. Of note, the baseline malnutrition and pre-operative steroid use in this cohort is lower than some other series [139, 144, 145].
Abelson et al. [144] reported trends in a comprehensive New York State database that showed a relatively steady rate of total abdominal colectomy as the initial operation between the pre-biologic (1995–2005) and the biologic (2006–2013) cohorts. Additionally, they reported an increase in three-stage operations over time. Finally, they argued for a sicker population at baseline given the increased comorbidities including anemia, weight loss and obesity. A qualitative study from Japan evaluated self-reported statistics using questionnaires to departments of surgery, in which only 14% of respondents reported an increase in subtotal colectomy since the introduction of infliximab and tacrolimus whereas 33% reported decrease and 53% were unchanged [142].
Given the disagreement in the single-center as well as population-based data, it is difficult to be definitive about trends in the choice of UC surgery since the introduction of biologics. Still, some initial conclusions are possible. The first is that the surgical population in the biologic era likely represents a ‘sicker’, more malnourished population that has likely had significant steroid exposure pre-operatively. Despite salvage strategies with biologics, non-elective surgery still represents 20%–30% of operations for UC and an initial total abdominal colectomy is the most common approach in these patients. Finally, if patients can be medically stabilized and go on to have elective surgery, a two-stage operation may be performed even in the setting of biologic use [143].
Are perioperative complications increased in patients with UC on biologics?
There is ongoing debate about the effect of biologic medication on perioperative outcomes in UC patients. Early studies in UC patients undergoing surgery pointed to an increased risk of complications—especially infectious complications—in patients with recent exposure (usually <12 weeks pre-operatively) to biologics [137, 146, 147]. However, in the decade since then, there have been over a dozen retrospective studies with varying results that have called this conclusion into question. Aside from the increased morbidity, readmissions, length of stay and costs associated with perioperative complications, the question of whether biologic treatment increases the perioperative complication rate is of particular importance to UC patients undergoing pouch reconstruction, given the well-characterized association between perioperative complications and worse long-term pouch function [148, 149].
Despite ongoing interest in this topic, no clear answer has emerged from the literature. Some authors suggest that the proposed increased risk of complications can be mitigated with an initial subtotal colectomy or three-stage approach: Gu et al. [150] report the Cleveland Clinic experience where biologic use in patients undergoing two-stage operation led to a 32% rate of pelvic sepsis at 1 year vs. 16% for patients not on biologics. Interestingly, this difference is not present 30 days post-operatively and the general increase in complications disappears when comparing patients undergoing subtotal colectomy as the initial operation (complication rates of 6 and 10% for biologic and non-biologic-treated groups). Nørgård et al. [151] presented the results from the Danish registry tracking over 1000 UC patients who underwent colectomy, of whom 199 were exposed to biologics. The authors found no difference in mortality, anastomotic leak, need for drainage or re-operation between the biologic- and non-biologic-treated groups. However, more than 80% of the biologic-treated group underwent an initial colectomy without proctectomy. Another single-center study by Hicks et al. [139] showed higher initial complications in patients undergoing two-stage vs three-stage operations (although only 29% and 18% of patients were on biologics at the time of operation); however, when cumulative complications of all operations were compared, the rates were similar. Interestingly, in this cohort, neither biologic usage nor steroid was associated with complications, but surgeon experience was.
Several meta-analyses and systematic reviews have examined the effect of biologics on perioperative outcomes, but the results are difficult to interpret [75, 78, 136, 152] (Table 5). Yang et al. [152] found no significant difference in infectious or non-infectious complications separately, but an increased risk of total complications (excluding one study) with biologic treatment was found (OR 1.80, 95% CI: 1.12–2.87). Both Narula et al. [75] and Billioud et al. [78] showed increased complications in IBD patients undergoing surgery on biologic therapy, but this finding lost its statistical significance when applied to UC patients only. Selvaggi et al. [136] performed a meta-analysis on UC surgery in general and specifically on primary IPAA surgery. When examining only primary IPAA surgery, they showed a significant increase in the risk of early complications and post-ileostomy closure in biologic-treated patients, with odds ratios of 4.12 (95% CI: 2.37–7.15) and 2.27 (95% CI: 1.27–4.05). However, similarly to other meta-analyses, when examining all UC patients undergoing any surgery, they found a non-significant trend towards a higher complication rate in UC patients on biologics (OR 1.19, 95% CI: 1.00–1.42) and, interestingly, a lower rate of surgical-site infections (OR 0.67, 95% CI: 0.45–0.99).
Table 5.
Summary of meta-analyses perioperative complications in patients with ulcerative colitis on biologic therapy
| Literature | Significant increase in infectious complications? | Significant increase in total complications? |
|---|---|---|
| Narula et al. (2013) [75] | No, OR = 1.39 (95% CI: 0.56–3.15) | No, OR = 1.10 (95% CI: 0.81–1.47) |
| Billioud et al. (2013) [78] | No, OR = 1.31 (95% CI: 0.55–3.07) | No, OR = 1.32 (95% CI: 0.94–1.84) |
| Selvaggi et al. (2015) [136] | No, OR = 1.12 (95% CI: 0.87–1.45) | All operations: No, OR = 1.19 (95% CI: 1.00–1.42) |
| IPAA-related: Yes, OR = 4.12 (95% CI: 2.37–7.15) | ||
| Ileostomy closure: Yes, OR = 2.27 (95% CI: 1.27–4.05) | ||
| Yang et al. (2010) [152] | No, OR = 2.24 (95% CI: 0.63–7.95) | Yes, OR = 1.80 (95% CI: 1.12–2.87) |
IPAA, ileal pouch-anal anastomosis; OR, odds ratio; CI, confidence interval.
In differentiating patient subgroups based on pre-operative biologic use, most authors grouped any exposure to biologic medication within 12 weeks of the operation into the ‘biologic’ group. However, this is likely too broad a category. Two recent papers have examined biologic drug levels in UC patients undergoing surgery with a reported biologic treatment history [82, 84]. Neither found a significant difference in perioperative outcomes between those UC patients with detectable vs undetectable levels, although, notably, both studies showed a significant proportion 71% (43/60) and 47% (9/19) of biologic-treated patients with undetectable levels at the time of operation. In studies where timing from last dose of biologic was examined, rather than serum levels, no association between increased complications and timing of last dose was noted [84, 153].
There are limited studies on non-TNF-α inhibitor biologics in perioperative outcomes for UC patients. Two groups examined the effect of vedolizumab on perioperative outcomes in IBD cohorts including UC and CD [85, 86]. Lightner et al. [85] found a significantly increased rate of post-operative complications in the vedolizumab-treated group (53%) versus the non-biologic group (33%) and the TNF-α inhibitor group (28%) in IBD patients, although most of the difference was in surgical-site infection. However, only 24% (22 patients) of the cohort had UC and subgroup analysis was not reported. Yamada et al. [86] performed a subgroup analysis of 24 UC patients treated with vedolizumab and demonstrated no increase in perioperative complications, and in fact saw a significant decrease in surgical-site infections. A recent meta-analysis of five studies also found no increase in total or infectious complications with pre-operative vedolizumab as compared to other TNF-α inhibitors or no biologic therapy [154].
Forming general conclusions about the effect of biologic medication on perioperative complications is not simple. The results are mixed and there is much heterogeneity within the patient populations in reported studies. Newer studies offer increasing granularity regarding biologic treatment and patient status, but the absolute numbers are small. Nevertheless, several themes emerge from the data. The first is that the association, if any, between biologic treatment and perioperative complications is less strong than in CD patients. Second, subtotal colectomy appears to be an effective way to avoid initial complications in UC patients being treated with biologics, but this does commit the patient to a longer, more expensive course that is not without risk of complications. Finally, at this time, it is very difficult to tell whether biologic treatment is an independent driver of patient outcomes or simply a marker of severe disease. Increasing rates of malnutrition, worsening rates of readmission and high rates of complications since the introduction of biologics tell us that the surgical population is getting sicker [9, 144]. However, there will clearly remain a population of UC patients who will inevitably need surgery and it is unclear whether this population’s outcomes will be worsened by direct effects of biologic treatment or indirectly by their use in prolonging time to surgical cure. Further studies with quantitative drug levels and more granular temporal associations will be necessary to gain resolution on these distinctions.
Conclusions
Biologic treatment has been proved to be a paradigm shift in the medical treatment of IBD patients. However, it does not appear to have substantially reduced the role of surgery in IBD care, with only minor decreases in surgical rates in recent years. Biologics may delay the necessity for surgery, especially in UC, potentially at the cost of increasingly severe malnourishment and chronic illness at the subsequent time of operation. As with all new treatments, there are nuances to the interaction of surgery and biologic treatment that require further study, including specifics pertaining to the quantitative effect of individual and combined drug regiments—including non-TNF-α biologic medication—on perioperative outcomes. Prospectively gathered data, with specific attention on not only treatments, but also patients’ perioperative condition, will allow surgeons to move beyond mixed data and personal preference to better, data-driven, surgical decision-making in the biologic era.
Funding
None
Conflicts of interests
Authors report no conflicts of interests.
References
- 1. Gunnarsson C, Chen J, Rizzo JA.. Direct health care insurer and out-of-pocket expenditures of inflammatory bowel disease: evidence from a US national survey. Dig Dis Sci 2012;57:3080–91. [DOI] [PubMed] [Google Scholar]
- 2. Mao R, Hu PJ.. The future of IBD therapy: where are we and where should we go next? Dig Dis 2016;34:175–9. [DOI] [PubMed] [Google Scholar]
- 3. Kappelman MD, Rifas-Shiman SL, Porter CQ. et al. Direct health care costs of Crohn’s disease and ulcerative colitis in US children and adults. Gastroenterology 2008;135:1907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Langholz E, Munkholm P, Davidsen M. et al. Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology 1994;107:3–11. [DOI] [PubMed] [Google Scholar]
- 5. Rizzo G, Pugliese D, Armuzzi A. et al. Anti-TNF alpha in the treatment of ulcerative colitis: a valid approach for organ-sparing or an expensive option to delay surgery? World J Gastroenterol 2014;20:4839–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bernstein CN, Loftus EV, Ng SC. et al. Hospitalisations and surgery in Crohn’s disease. Gut 2012;61:622–9. [DOI] [PubMed] [Google Scholar]
- 7. Mandel MD, Miheller P, Müllner K. et al. Have biologics changed the natural history of Crohn’s disease? Dig Dis 2014;32:351–9. [DOI] [PubMed] [Google Scholar]
- 8. Paramsothy S, Rosenstein AK, Mehandru S. et al. The current state of the art for biological therapies and new small molecules in inflammatory bowel disease. Mucosal Immunol 2018;11:1558–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hatch QM, Ratnaparkhi R, Althans A. et al. Is modern medical management changing ultimate patient outcomes in inflammatory bowel disease? J Gastrointest Surg 2016;20:1867–73. [DOI] [PubMed] [Google Scholar]
- 10. Deiana S, Gabbani T, Annese V. et al. Biosimilars in inflammatory bowel disease: a review of post-marketing experience. World J Gastroenterol 2017;23:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zheng MK, Shih DQ, Chen GC.. Insights on the use of biosimilars in the treatment of inflammatory bowel disease. World J Gastroenterol 2017;23:1932–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kinch MS. An overview of FDA-approved biologics medicines. Drug Discov Today 2015;20:393–8. [DOI] [PubMed] [Google Scholar]
- 13. Targan S, Hanauer S, Van Deventer S. et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease: Crohn’s Disease cA2 Study Group. N Engl J Med 1997;337:1029–35. [DOI] [PubMed] [Google Scholar]
- 14. Schreiber S, Rutgeerts P, Fedorak RN. et al. A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn’s disease. Gastroenterlogy 2005;129:807–18. [DOI] [PubMed] [Google Scholar]
- 15. Jeuring SF, Bours PH, Zeegers MP. et al. Disease outcome of ulcerative colitis in an era of changing treatment strategies: results from the Dutch population-based IBDSL cohort. J Crohns Colitis 2015;9:837–45. [DOI] [PubMed] [Google Scholar]
- 16. Rönnblom A, Holmström T, Tanghöj H. et al. Low colectomy rate five years after diagnosis of ulcerative colitis. Results from a prospective population-based cohort in Sweden (ICURE) diagnosed during 2005–2009. Scand J Gastroenterol 2016;51:1339–44. [DOI] [PubMed] [Google Scholar]
- 17. Nguyen GC, Nugent Z, Shaw S. et al. Outcomes of patients with Crohn’s disease improved from 1988 to 2008 and were associated with increased specialist care. Gastroenterology 2011;141:90–7. [DOI] [PubMed] [Google Scholar]
- 18. Rungoe C, Langholz E, Andersson M. et al. Changes in medical treatment and surgery rates in inflammatory bowel disease: a nationwide cohort study 1979–2011. Gut 2014;63:1607–16. [DOI] [PubMed] [Google Scholar]
- 19. Yu H, MacIsaac D, Wong JJ. et al. Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment Pharmacol Ther 2018;47:364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hancock L, Mortensen NJ.. How often do IBD patients require resection of their intestine? Inflamm Bowel Dis 2008;14 (Suppl 2):S68–9. [DOI] [PubMed] [Google Scholar]
- 21. Bouguen G, Peyrin-Biroulet L.. Surgery for adult Crohn’s disease: what is the actual risk? Gut 2011;60:1178–81. [DOI] [PubMed] [Google Scholar]
- 22. Frolkis AD, Dykeman J, Negrón ME. et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology 2013;145:996–1006. [DOI] [PubMed] [Google Scholar]
- 23. Hanauer SB, Feagan BG, Lichtenstein GR. et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002;359:1541–9. [DOI] [PubMed] [Google Scholar]
- 24. Colombel JF, Sandborn WJ, Rutgeerts P. et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 2007;132:52–65. [DOI] [PubMed] [Google Scholar]
- 25. Rutgeerts P, Feagan BG, Lichtenstein GR. et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology 2004;126:402–13. [DOI] [PubMed] [Google Scholar]
- 26. Feagan BG, Panaccione R, Sandborn WJ. et al. Effects of adalimumab therapy on incidence of hospitalization and surgery in Crohn’s disease: results from the CHARM study. Gastroenterology 2008;135:1493–9. [DOI] [PubMed] [Google Scholar]
- 27. Vind I, Riis L, Jess T. et al. Increasing incidences of inflammatory bowel disease and decreasing surgery rates in Copenhagen City and County, 2003–2005: a population-based study from the Danish Crohn colitis database. Am J Gastroenterol 2006;101:1274–82. [DOI] [PubMed] [Google Scholar]
- 28. Jones DW, Finlayson SRG.. Trends in surgery for Crohn’s disease in the era of infliximab. Ann Surg 2010;252:307–12. [DOI] [PubMed] [Google Scholar]
- 29. Lazarev M, Ullman T, Schraut WH. et al. Small bowel resection rates in Crohn’s disease and the indication for surgery over time: experience from a large tertiary care center. Inflamm Bowel Dis 2010;16:830.. [DOI] [PubMed] [Google Scholar]
- 30. Ramadas AV, Gunesh S, Thomas GA. et al. Natural history of Crohn’s disease in a population-based cohort from Cardiff (1986-2003): a study of changes in medical treatment and surgical resection rates. Gut 2010;59:1200–6. [DOI] [PubMed] [Google Scholar]
- 31. Peyrin-Biroulet L, Salleron J, Filippi J. et al. Anti-TNF monotherapy for Crohn’s disease: a 13-year multicentre experience. J Crohns Colitis 2016;10:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Slattery E, Keegan D, Hyland J. et al. Surgery, Crohn’s disease, and the biological era: has there been an impact? J Clin Gastroenterol 2011;45:691–3. [DOI] [PubMed] [Google Scholar]
- 33. Peyrin-Biroulet L, Oussalah A, Williet N. et al. Impact of azathioprine and tumour necrosis factor antagonists on the need for surgery in newly diagnosed Crohn’s disease. Gut 2011;60:930–6. [DOI] [PubMed] [Google Scholar]
- 34. Schnitzler F, Fidder H, Ferrante M. et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: results from a single-centre cohort. Gut 2009;58:492–500. [DOI] [PubMed] [Google Scholar]
- 35. Eshuis EJ, Peters CP, van Bodegraven AA. et al. Ten years of infliximab for Crohn’s disease: outcome in 469 patients from 2 tertiary referral centers. Inflamm Bowel Dis 2013;19:1622–30. [DOI] [PubMed] [Google Scholar]
- 36. Alzafiri R, Holcroft CA, Malolepszy P. et al. Infliximab therapy for moderately severe Crohn’s disease and ulcerative colitis: a retrospective comparison over 6 years. Clin Exp Gastroenterol 2011;4:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caviglia R, Ribolsi M, Rizzi M. et al. Maintenance of remission with infliximab in inflammatory bowel disease: efficacy and safety long-term follow-up. World J Gastroenterol 2007;13:5238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ljung T, Karlén P, Schmidt D. et al. Infliximab in inflammatory bowel disease: clinical outcome in a population based cohort from Stockholm County. Gut 2004;53:849–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Feagan BG, Greenberg GR, Wild G. et al. Treatment of active Crohn’s disease with MLN0002, a humanized antibody to the α4β7 integrin. Clin Gastroenterol Hepatol 2008;6:1370–7. [DOI] [PubMed] [Google Scholar]
- 40. Yokoyama K, Yamazaki K, Katafuchi M. et al. A retrospective claims database study on drug utilization in Japanese patients with Crohn’s disease treated with adalimumab or infliximab. Adv Ther 2016;33:1947–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Frolkis AD, Lipton DS, Fiest KM. et al. Cumulative incidence of second intestinal resection in Crohn’s disease: a systematic review and meta-analysis of population-based studies. Am J Gastroenterol 2014;109:1739–48. [DOI] [PubMed] [Google Scholar]
- 42. An V, Cohen L, Lawrence M. et al. Early surgery in Crohn’s disease a benefit in selected cases. World J Gastrointest Surg 2016;8:492.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aratari A, Papi C, Leandro G. et al. Early versus late surgery for ileo-caecal Crohn’s disease. Aliment Pharmacol Ther 2007;26:1303–12. [DOI] [PubMed] [Google Scholar]
- 44. Sorrentino D, Paviotti A, Terrosu G. et al. Low-dose maintenance therapy with infliximab prevents postsurgical recurrence of Crohn’s disease. Clin Gastroenterol Hepatol 2010;8:591–9.e1. [DOI] [PubMed] [Google Scholar]
- 45. Savarino E, Bodini G, Dulbecco P. et al. Adalimumab is more effective than azathioprine and mesalamine at preventing postoperative recurrence of Crohn’s disease: a randomized controlled trial. Am J Gastroenterol 2013;108:1731–42. [DOI] [PubMed] [Google Scholar]
- 46. Yoshida K, Fukunaga K, Ikeuchi H. et al. Scheduled infliximab monotherapy to prevent recurrence of Crohn’s disease following ileocolic or ileal resection: a 3-year prospective randomized open trial. Inflamm Bowel Dis 2012;18:1617–23. [DOI] [PubMed] [Google Scholar]
- 47. Regueiro M, Kip KE, Baidoo L. et al. Postoperative therapy with infliximab prevents long-term crohn’s disease recurrence. Clin Gastroenterol Hepatol 2014;12:1494–502.e1. [DOI] [PubMed] [Google Scholar]
- 48. Magro F, Santos-Antunes J, Vilas-Boas F. et al. Crohn’s disease outcome in patients under azathioprine: a tertiary referral center experience. J Crohns Colitis 2014;8:617–25. [DOI] [PubMed] [Google Scholar]
- 49. López-Sanromán A, Vera-Mendoza I, Domènech E. et al. Adalimumab vs azathioprine in the prevention of postoperative Crohn’s disease recurrence. A GETECCU randomized trial. J Crohns Colitis 2017;11:1293–1301 [DOI] [PubMed] [Google Scholar]
- 50. Campbell JP, Vaughn BP.. Optimal delivery of follow-up care after surgery for Crohn’s disease: current perspectives. Clin Exp Gastroenterol 2016;9:237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Regueiro M, Feagan BG, Zou B. et al. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn’s disease after ileocolonic resection. Gastroenterology 2016;150:1568–78. [DOI] [PubMed] [Google Scholar]
- 52. Rutgeerts P. Review article: recurrence of Crohn’s disease after surgery—the need for treatment of new lesions. Aliment Pharmacol Ther 2006;24 (Suppl 3):29–32. [DOI] [PubMed] [Google Scholar]
- 53. De Cruz P, Kamm MA, Hamilton AL. et al. Crohn’s disease management after intestinal resection: a randomised trial. Lancet 2015;385:1406–17. [DOI] [PubMed] [Google Scholar]
- 54. Bordeianou L, Stein SL, Ho VP. et al. Immediate versus tailored prophylaxis to prevent symptomatic recurrences after surgery for ileocecal Crohn’s disease? Surgery 2011;149:72–8. [DOI] [PubMed] [Google Scholar]
- 55. De Cruz P, Kamm MA, Prideaux L. et al. Postoperative recurrent luminal Crohn’s disease: a systematic review. Inflamm Bowel Dis 2012;18:758–77. [DOI] [PubMed] [Google Scholar]
- 56. Nguyen GC, Loftus EV, Hirano I. et al. American gastroenterological association institute guideline on the management of Crohn’s disease after surgical resection. Gastroenterology 2017;152:271–5. [DOI] [PubMed] [Google Scholar]
- 57. Regueiro M, El-Hachem S, Kip KE. et al. Postoperative infliximab is not associated with an increase in adverse events in Crohn’s disease. Dig Dis Sci 2011;56:3610–5. [DOI] [PubMed] [Google Scholar]
- 58. Pittet V, Froehlich F, Maillard MH. et al. When do we dare to stop biological or immunomodulatory therapy for Crohn’s disease? Results of a multidisciplinary European expert panel. J Crohn’s Colitis 2013;7:820–6. [DOI] [PubMed] [Google Scholar]
- 59. Papamichael K, Vermeire S.. Withdrawal of anti-tumour necrosis factor α therapy in inflammatory bowel disease. World J Gastroenterol 2015;21:4773–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Torres J, Boyapati RK, Kennedy NA. et al. Systematic review of effects of withdrawal of immunomodulators or biologic agents from patients with inflammatory bowel disease. Gastroenterology 2015;149:1716–30. [DOI] [PubMed] [Google Scholar]
- 61. Herfarth HH. Anti-tumor necrosis factor therapy to prevent Crohn’s disease recurrence after surgery. Clin Gastroenterol Hepatol 2014;12:1503–6. [DOI] [PubMed] [Google Scholar]
- 62. Beddy D, Dozois EJ, Pemberton JH.. Perioperative complications in inflammatory bowel disease. Inflamm Bowel Dis 2011;17:1610–9. [DOI] [PubMed] [Google Scholar]
- 63. Patel KV, Darakhshan AA, Griffin N. et al. Patient optimization for surgery relating to Crohn’s disease. Nat Rev Gastroenterol Hepatol 2016;13:707–19. [DOI] [PubMed] [Google Scholar]
- 64. Navaneethan U, Parasa S, Venkatesh PG. et al. Impact of inflammatory bowel disease on post-cholecystectomy complications and hospitalization costs: a Nationwide Inpatient Sample study. J Crohns Colitis 2013;7:e164–70. [DOI] [PubMed] [Google Scholar]
- 65. Yamamoto T, Allan RN, Keighley MR.. Risk factors for intra-abdominal sepsis after surgery in Crohn’s disease. Dis Colon Rectum 2000;43:1141–5. [DOI] [PubMed] [Google Scholar]
- 66. Huang W, Tang Y, Nong L. et al. Risk factors for postoperative intra-abdominal septic complications after surgery in Crohn’s disease: a meta-analysis of observational studies. J Crohns Colitis 2015;9:293–301. [DOI] [PubMed] [Google Scholar]
- 67. Mooney D, O’Reilly M, Gamelli R.. Tumor necrosis factor and wound healing. Ann Surg 1990;221:124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ploug T, Andersen K, Hansen K. et al. Influence of adalimumab treatment on anastomotic strength, degree of inflammation, and collagen formation: an experimental study on the small intestine of rabbits. Inflamm Bowel Dis 2013;19:254–8. [DOI] [PubMed] [Google Scholar]
- 69. Frostberg E, Ström P, Gerke O. et al. Infliximab’s influence on anastomotic strength and degree of inflammation in intestinal surgery in a rabbit model. BMC Surg 2014;14:23.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lightner AL, Shen B.. Perioperative use of immunosuppressive medications in patients with Crohn’s disease in the new ‘biological era’. Gastroenterol Rep (Oxf) 2017;5:165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Paulson EC. Biologic therapy and surgery for Crohn disease. Clin Colon Rectal Surg 2013;26:128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zaghiyan K, McGovern D, Fleshner P.. Should biologic agents be stopped before surgery for inflammatory bowel disease? Expert Rev Gastroenterol Hepatol 2015;9:269–72. [DOI] [PubMed] [Google Scholar]
- 73. Chang MI, Cohen BL, Greenstein AJ.. A review of the impact of biologics on surgical complications in Crohn’s disease. Inflamm Bowel Dis 2015;21:1472–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Holubar SD, Holder-Murray J, Flasar M. et al. Anti-tumor necrosis factor-alpha antibody therapy management before and after intestinal surgery for inflammatory bowel disease: a CCFA position paper. Inflamm Bowel Dis 2015;21:2658–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Narula N, Charleton D, Marshall JK.. Meta-analysis: peri-operative anti-TNFα treatment and post-operative complications in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2013;37:1057–64. [DOI] [PubMed] [Google Scholar]
- 76. El-Hussuna A, Krag A, Olaison G. et al. The effect of anti-tumor necrosis factor alpha agents on postoperative anastomotic complications in Crohn’s disease: a systematic review. Dis Colon Rectum 2013;56:1423–33. [DOI] [PubMed] [Google Scholar]
- 77. Kopylov U, Ben-Horin S, Zmora O. et al. Anti-tumor necrosis factor and postoperative complications in Crohn’s disease: systematic review and meta-analysis. Inflamm Bowel Dis 2012;18:2404–13. [DOI] [PubMed] [Google Scholar]
- 78. Billioud V, Ford AC, Tedesco ED. et al. Preoperative use of anti-TNF therapy and postoperative complications in inflammatory bowel diseases: a meta-analysis. J Crohns Colitis 2013;7:853–67. [DOI] [PubMed] [Google Scholar]
- 79. Waterland P, Athanasiou T, Patel H.. Post-operative abdominal complications in Crohn’s disease in the biological era: systematic review and meta-analysis. World J Gastrointest Surg 2016;8:274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yang ZP, Hong L, Wu Q. et al. Preoperative infliximab use and postoperative complications in Crohn’s disease: a systematic review and meta-analysis. Int J Surg 2014;12:224–30. [DOI] [PubMed] [Google Scholar]
- 81. Sheasgreen C, Nguyen GC.. The evolving evidence for therapeutic drug monitoring of monoclonal antibodies in inflammatory bowel disease. Curr Gastroenterol Rep 2017;19:19.. [DOI] [PubMed] [Google Scholar]
- 82. Lau C, Dubinsky M, Melmed G. et al. The impact of preoperative serum anti-TNFα therapy levels on early postoperative outcomes in inflammatory bowel disease surgery. Ann Surg 2015;261:487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fumery M, Seksik P, Auzolle C. et al. REMIND study group investigators. Postoperative complications after ileocecal resection in Crohn’s disease: a prospective study from the REMIND group. Am J Gastroenterol 2016;112:337–45. [DOI] [PubMed] [Google Scholar]
- 84. Waterman M, Xu W, Dinani A. et al. Preoperative biological therapy and short-term outcomes of abdominal surgery in patients with inflammatory bowel disease. Gut 2013;62:387–94. [DOI] [PubMed] [Google Scholar]
- 85. Lightner AL, Raffals LE, Mathis KL. et al. Postoperative outcomes in vedolizumab-treated patients undergoing abdominal operations for inflammatory bowel disease. J Crohns Colitis 2017;11:185–90. [DOI] [PubMed] [Google Scholar]
- 86. Yamada A, Komaki Y, Patel N. et al. Risk of postoperative complications among inflammatory bowel disease patients treated preoperatively with vedolizumab. Am J Gastroenterol 2017;112:1423–9. [DOI] [PubMed] [Google Scholar]
- 87. Schwartz DA, Herdman CR.. Review article: the medical treatment of Crohn’s perianal fistulas. Aliment Pharmacol Ther 2004;19:953–67. [DOI] [PubMed] [Google Scholar]
- 88. Present D. Review article: the efficacy of infliximab in Crohn’s disease—healing of fistulae. Aliment Pharmacol Ther 1999;13 (Suppl 4):23–8; dicussion 38. [DOI] [PubMed] [Google Scholar]
- 89. Chhaya V, Saxena S, Cecil E. et al. Emerging trends and risk factors for perianal surgery in Crohn’s disease: a 20-year national population-based cohort study. Eur J Gastroenterol Hepatol 2016;28:890–5. [DOI] [PubMed] [Google Scholar]
- 90. Eglinton T, Reilly M, Chang C. et al. Ileal disease is associated with surgery for perianal disease in a population-based Crohn’s disease cohort. Br J Surg 2010;97:1103–9. [DOI] [PubMed] [Google Scholar]
- 91. Schwartz DA, Loftus EV, Tremaine WJ. et al. The natural history of fistulizing Crohn’s disease. Gastroenterlogy 2002;122:875–80. [DOI] [PubMed] [Google Scholar]
- 92. Williamson PR, Hellinger MD, Larach SW. et al. Twenty-year review of the surgical management of perianal Crohn’s disease. Dis Colon Rectum 1995;38:389–92. [DOI] [PubMed] [Google Scholar]
- 93. Mueller MH, Geis M, Glatzle J. et al. Risk of fecal diversion in complicated perianal Crohn’s disease. J Gastrointest Surg 2007;11:529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Present D, Rutgeerts P, Targan S. et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med 1999;340:1398–405. [DOI] [PubMed] [Google Scholar]
- 95. Sands BE, Anderson FH, Bernstein CN. et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med 2004;350:876–85. [DOI] [PubMed] [Google Scholar]
- 96. Colombel J, Schwartz DA, Sandborn WJ. et al. Adalimumab for the treatment of fistulas in patients with Crohn’s disease. Gut 2009;58:940–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Papamichael K, Cheifetz AS.. Defining and predicting deep remission in patients with perianal fistulizing Crohn’s disease on anti-tumor necrosis factor therapy. World J Gastroenterol 2017;23:6197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yassin NA, Askari A, Warusavitarne J. et al. Alimentary pharmacology and therapeutics systematic review: the combined surgical and medical treatment of fistulising perianal Crohn’s disease. Aliment Pharmacol Ther 2014;40:741–9. [DOI] [PubMed] [Google Scholar]
- 99. El-Gazzaz G, Hull T, Church JM.. Biological immunomodulators improve the healing rate in surgically treated perianal Crohn’s fistulas. Colorectal Dis 2012;14:1217–23. [DOI] [PubMed] [Google Scholar]
- 100. Gaertner WB, Decanini A, Mellgren A. et al. Does infliximab infusion impact results of operative treatment for Crohn’s perianal fistulas? Dis Colon Rectum 2007;50:1754–60. [DOI] [PubMed] [Google Scholar]
- 101. Haennig A, Staumont G, Lepage B. et al. The results of seton drainage combined with anti-TNFα therapy for anal fistula in Crohn’s disease. Colorectal Dis 2015;17:311–9. [DOI] [PubMed] [Google Scholar]
- 102. Bouguen G, Siproudhis L, Gizard E. et al. Long-term outcome of perianal fistulizing Crohn’s disease treated with infliximab. Clin Gastroenterol Hepatol 2013;11:975–81.e4. [DOI] [PubMed] [Google Scholar]
- 103. Kelley KA, Kaur T, Tsikitis V.. Perianal Crohn’s disease: challenges and solutions. Clin Exp Gastroenterol 2017;8:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Marzo M, Felice C, Pugliese D. et al. Management of perianal fistulas in Crohn’s disease: an up- to-date review. World J Gastroenterol 2015;21:1394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Poggioli G, Laureti S, Pierangeli F. et al. Local injection of infliximab for the treatment of perianal Crohn’s disease. Dis Colon Rectum 2005;48:768–74. [DOI] [PubMed] [Google Scholar]
- 106. Tonelli F, Giudici F, Asteria C.. Effectiveness and safety of local adalimumab injection in patients with fistulizing perianal Crohn’s disease: a pilot study. Dis Colon Rectum 2012;55:870–5. [DOI] [PubMed] [Google Scholar]
- 107. Singh S, Ding NS, Mathis KL. et al. Systematic review with meta-analysis: faecal diversion for management of perianal Crohn’s disease. Aliment Pharmacol Ther 2015;42:783–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ej De G, Buskens CJ, Ponsioen CY. et al. Multimodal treatment of perianal fistulas in Crohn’s disease: seton versus anti-TNF versus advancement plasty (PISA): study protocol for a randomized controlled trial. Trials 2015;20:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Manetti N, Bagnoli S, Rogai F. et al. Disease course and colectomy rate of ulcerative colitis: a follow-up cohort study of a referral center in Tuscany. Inflamm Bowel Dis 2016;22:1945–53. [DOI] [PubMed] [Google Scholar]
- 110. Cohen JL, Strong SA, Hyman NH. et al. Practice parameters for the surgical treatment of ulcerative colitis. Dis Colon Rectum 2005;48:1997–2009. [DOI] [PubMed] [Google Scholar]
- 111. Rutgeerts PJ, Sandborn WJ, Feagan BG. et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;355:2462–76. [DOI] [PubMed] [Google Scholar]
- 112. Sandborn WJ, Rutgeerts P, Feagan BG. et al. Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology 2009;137:1250–60. [DOI] [PubMed] [Google Scholar]
- 113. Järnerot G, Hertervig E, Friis-Liby I. et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterlogy 2005;128:1805–11. [DOI] [PubMed] [Google Scholar]
- 114. Biondi A, Zoccali M, Costa S. et al. Surgical treatment of ulcerative colitis in the biologic therapy era. World J Gastroenterol 2012;18:1861–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Gustavsson A, Järnerot G, Hertervig E. et al. Clinical trial: colectomy after rescue therapy in ulcerative colitis—3-year follow-up of the Swedish-Danish controlled infliximab study. Aliment Pharmacol Ther 2010;32:984–9. [DOI] [PubMed] [Google Scholar]
- 116. Williams JG, Alam FM, Alrubaiy L. et al. Comparison of infliximab and ciclosporin in steroid resistant ulcerative colitis: pragmatic randomised trial and economic evaluation (CONSTRUCT). Health Technol Assess 2016;20:1–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Laharie D, Bourreille A, Branche J. et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet 2012;380:1909–15. [DOI] [PubMed] [Google Scholar]
- 118. Laharie D, Bourreille A, Branche J. et al. Long-term outcome of patients with steroid- refractory acute severe UC treated with ciclosporin or infliximab. Gut 2018;67:237–43. [DOI] [PubMed] [Google Scholar]
- 119. Aratari A, Papi C, Clemente V. et al. Colectomy rate in acute severe ulcerative colitis in the infliximab era. Dig Liver Dis 2008;40:821–6. [DOI] [PubMed] [Google Scholar]
- 120. Ferrante M, Vermeire S, Fidder H. et al. Long-term outcome after infliximab for refractory ulcerative colitis. J Crohns Colitis 2008;2:219–25. [DOI] [PubMed] [Google Scholar]
- 121. Oussalah A, Evesque L, Laharie D. et al. A multicenter experience with infliximab for ulcerative colitis: outcomes and predictors of response. Am J Gastroenterol 2010;105:2617–25. [DOI] [PubMed] [Google Scholar]
- 122. Sjöberg M, Magnuson A, Björk J. et al. Infliximab as rescue therapy in hospitalised patients with steroid-refractory acute ulcerative colitis: a long-term follow-up of 211 Swedish patients. Aliment Pharmacol Ther 2013;38:377–87. [DOI] [PubMed] [Google Scholar]
- 123. Angelison A, Almer S, Eriksson A. et al. Alimentary pharmacology and therapeutics long-term outcome of infliximab treatment in chronic active ulcerative colitis: a Swedish multicentre study of 250 patients. Aliment Pharmacol Ther 2016;45:519–32. [DOI] [PubMed] [Google Scholar]
- 124. Teisner AS, Ainsworth MA, Brynskov J.. Long-term effects and colectomy rates in ulcerative colitis patients treated with infliximab: a Danish single center experience. Scand J Gastroenterol 2010;45:1457–63. [DOI] [PubMed] [Google Scholar]
- 125. Baki E, Zwickel P, Zawierucha A. et al. Real-life outcome of anti-tumor necrosis factor α in the ambulatory treatment of ulcerative colitis. World J Gastroenterol 2015;21:3282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Jakobovits S, Jewell DP, Travis SP.. Infliximab for the treatment of ulcerative colitis: outcomes in Oxford from 2000 to 2006. Aliment Pharmacol Ther 2007;25:1055–60. [DOI] [PubMed] [Google Scholar]
- 127. Mortensen C, Caspersen S, Lyhne N. et al. Treatment of acute ulcerative colitis with infliximab, a retrospective study from three Danish hospitals. J Crohns Colitis 2011;5:28–33. [DOI] [PubMed] [Google Scholar]
- 128. Costa J, Magro F, Caldeira D. et al. Infliximab reduces hospitalizations and surgery interventions in patients with inflammatory bowel disease. Inflamm Bowel Dis 2013;19:2098–110. [DOI] [PubMed] [Google Scholar]
- 129. Reich KM, Chang H, Rezaie A. et al. The incidence rate of colectomy for medically refractory ulcerative colitis has declined in parallel with increasing anti-TNF use: a time-trend study. Aliment Pharmacol Ther 2014;40:629–38. [DOI] [PubMed] [Google Scholar]
- 130. Kin C, Bundorf MK.. As infliximab use for ulcerative colitis has increased, so has the rate of surgical resection. J Gastrointest Surg 2017;21:1159–65. [DOI] [PubMed] [Google Scholar]
- 131. Solberg IC, Lygren I, Jahnsen J. et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol 2009;44:431–40. [DOI] [PubMed] [Google Scholar]
- 132. Al-Darmaki A, Hubbard J, Seow CH. et al. Clinical predictors of the risk of early colectomy in ulcerative colitis: a population-based study. Inflamm Bowel Dis 2017;23:1272–7. [DOI] [PubMed] [Google Scholar]
- 133. Targownik LE, Singh H, Nugent Z. et al. The epidemiology of colectomy in ulcerative colitis: results from a population-based cohort. Am J Gastroenterol 2012;107:1228–35. [DOI] [PubMed] [Google Scholar]
- 134. Sofo L, Caprino P, Sacchetti F. et al. Restorative proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis: a narrative review. World J Gastrointest Surg 2016;8:556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Cima RR. Timing and indications for colectomy in chronic ulcerative colitis: surgical consideration. Dig Dis 2010;28:501–7. [DOI] [PubMed] [Google Scholar]
- 136. Selvaggi F, Pellino G, Canonico S. et al. Effect of preoperative biologic drugs on complications and function after restorative proctocolectomy with primary ileal pouch formation: systematic review and meta-analysis. Inflamm Bowel Dis 2015;21:79–92. [DOI] [PubMed] [Google Scholar]
- 137. Mor IJ, Vogel JD, Da Luz Moreira A. et al. Infliximab in ulcerative colitis is associated with an increased risk of postoperative complications after restorative proctocolectomy. Dis Colon Rectum 2008;51:1202–7. [DOI] [PubMed] [Google Scholar]
- 138. Pandey S, Luther G, Umanskiy K. et al. Minimally invasive pouch surgery for ulcerative colitis: is there a benefit in staging? Dis Colon Rectum 2011;54:306–10. [DOI] [PubMed] [Google Scholar]
- 139. Hicks CW, Hodin RA, Bordeianou L.. Possible overuse of 3-stage procedures for active ulcerative colitis. JAMA Surg 2013;148:658–64. [DOI] [PubMed] [Google Scholar]
- 140. Ferrante M, D'hoore A, Vermeire S. et al. Corticosteroids but not infliximab increase short-term postoperative infectious complications in patients with ulcerative colitis. Inflamm Bowel Dis 2009;15:1062–70. [DOI] [PubMed] [Google Scholar]
- 141. Geltzeiler CB, Lu KC, Diggs BS. et al. Initial surgical management of ulcerative colitis in the biologic era. Dis Colon Rectum 2014;57:1358–63. [DOI] [PubMed] [Google Scholar]
- 142. Kimura H, Takahashi K, Futami K. et al. Has widespread use of biologic and immunosuppressant therapy for ulcerative colitis affected surgical trends? Results of a questionnaire survey of surgical institutions in Japan. Surg Today 2016;46:930–8. [DOI] [PubMed] [Google Scholar]
- 143. Bikhchandani J, Polites SF, Wagie AE. et al. National trends of 3- versus 2-stage restorative proctocolectomy for chronic ulcerative colitis. Dis Colon Rectum 2015;58:199–204. [DOI] [PubMed] [Google Scholar]
- 144. Abelson JS, Michelassi F, Mao J. et al. Higher surgical morbidity for ulcerative colitis patients in the era of biologics. Ann Surg 2018;268:311–7. [DOI] [PubMed] [Google Scholar]
- 145. Gu J, Stocchi L, Ashburn J. et al. Total abdominal colectomy vs. restorative total proctocolectomy as the initial approach to medically refractory ulcerative colitis. Int J Colorectal Dis 2017;32:1215–22. [DOI] [PubMed] [Google Scholar]
- 146. Selvasekar CR, Cima RR, Larson DW. et al. Effect of infliximab on short-term complications in patients undergoing operation for chronic ulcerative colitis. J Am Coll Surg 2007;204:956–62. [DOI] [PubMed] [Google Scholar]
- 147. Schluender SJ, Ippoliti A, Dubinsky M. et al. Does infliximab influence surgical morbidity of ileal pouch-anal anastomosis in patients with ulcerative colitis? Dis Colon Rectum 2007;50:1747–53. [DOI] [PubMed] [Google Scholar]
- 148. Kiely JM, Fazio VW, Remzi FH. et al. Pelvic sepsis after IPAA adversely affects function of the pouch and quality of life. Dis Colon Rectum 2012;55:387–92. [DOI] [PubMed] [Google Scholar]
- 149. McCombie A, Lee Y, Vanamala R. et al. Early postoperative complications have long-term impact on quality of life after restorative proctocolectomy. Medicine (Baltimore) 2016;95:e3966.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Gu J, Remzi FH, Shen B. et al. Operative strategy modifies risk of pouch-related outcomes in patients with ulcerative colitis on preoperative anti-tumor necrosis factor-α therapy. Dis Colon Rectum 2013;56:1243–52. [DOI] [PubMed] [Google Scholar]
- 151. Nørgård BM, Nielsen J, Qvist N. et al. Pre-operative use of anti-TNF-α agents and the risk of post-operative complications in patients with ulcerative colitis—a nationwide cohort study. Aliment Pharmacol Ther 2012;35:1301–9. [DOI] [PubMed] [Google Scholar]
- 152. Yang Z, Wu Q, Wu K. et al. Meta-analysis: pre-operative infliximab treatment and short-term post-operative complications in patients with ulcerative colitis. Aliment Pharmacol Ther 2010;31:486–92. [DOI] [PubMed] [Google Scholar]
- 153. Alsaleh A, Gaidos JKJ, Kang L. et al. Timing of last preoperative dose of infliximab does not increase postoperative complications in inflammatory bowel disease patients. Dig Dis Sci 2016;61:2602–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Law CCY, Narula A, Lightner AL. et al. Systematic review and meta-analysis: preoperative vedolizumab treatment and postoperative complications in patients with inflammatory bowel disease. J Crohns Colitis 2018;12:538–45. [DOI] [PubMed] [Google Scholar]