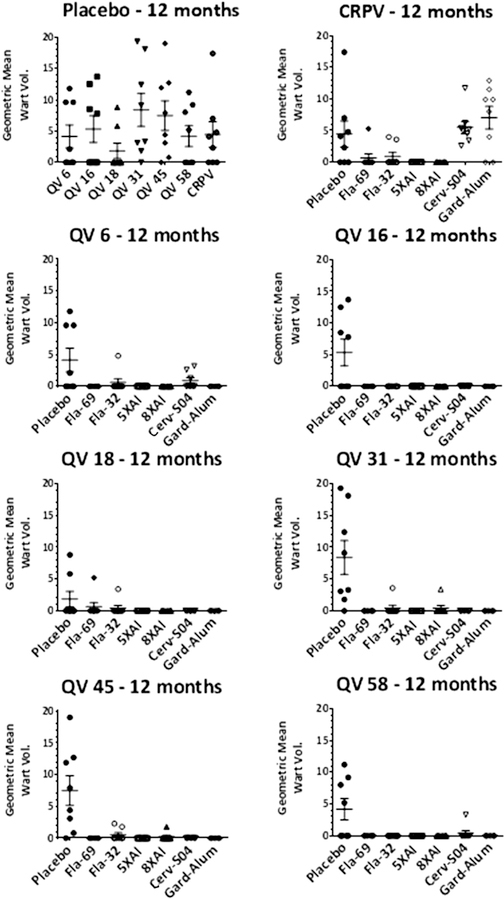
Fig. 3.
Skin challenge of rabbits at 12 months post vaccination with HPV6, 16, 18, 31, 45 and 58 quazivirions and CRPV. Rabbits were immunized i.m. three times with placebo (not shown) or 125 µg of the following antigen preparations: Fla69, Fla32, α11–88×5 formulated with aluminum phosphate (5XAl); α11–88×8 formulated with aluminum phosphate (8XAl). As a positive control, additional rabbits were vaccinated with 3 full human doses of Cervarix® which includes HPV 16 and 18 L1 VLP formulated with the ASO4 adjuvant (Cerv-SO4), or GARDASIL® comprising HPV6, 11, 16 and 18 L1 VLP formulated on a proprietary alum-based adjuvant (Gard-Alum). One year later the rabbits were challenged with HPV6, 16, 18, 31, 45 and 58 quazivirions and CRPV and followed for 8 weeks for papilloma growth.