Fig. 4.
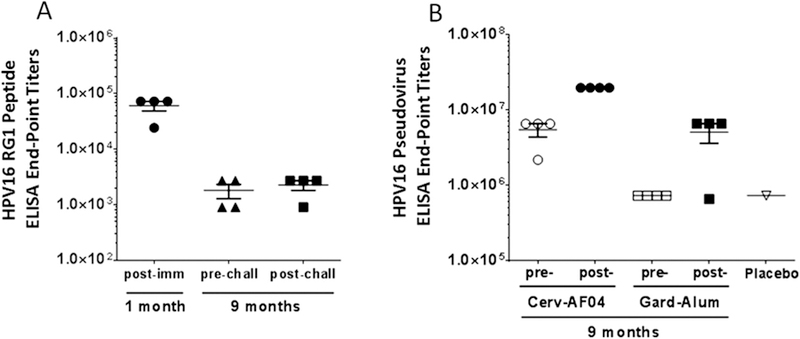
Assessment of anamnestic response upon challenge 9 months post-immunization with either Fla69, or Gardasil (Gard-Alum) or Cervarix (Cerv-AS04) immunized i.m. once a month for three months. (A) HPV16 RG1 (aa 17–38) peptide ELISA with rabbit serum from 1 month (study day 28) post-vaccination with Fla69 and 9 months (study day 310) post-vaccination with Fla69 and 1 week post-cutaneous challenge with HPV6, 16, 18, 31, 45 and 58 quazivirions and CRPV (study day 317). Since there was no significant difference in titer one week after challenge, which suggests no detectable systemic anamnestic response to this epitope after skin challenge. (B) HPV16 pseudovirus ELISA with rabbit serum from 9 months (study day 310) post-vaccination with Cervarix and Gardasil and post-challenge (study day 317) demonstrating an anamnestic response to HPV16 L1 VLP.
