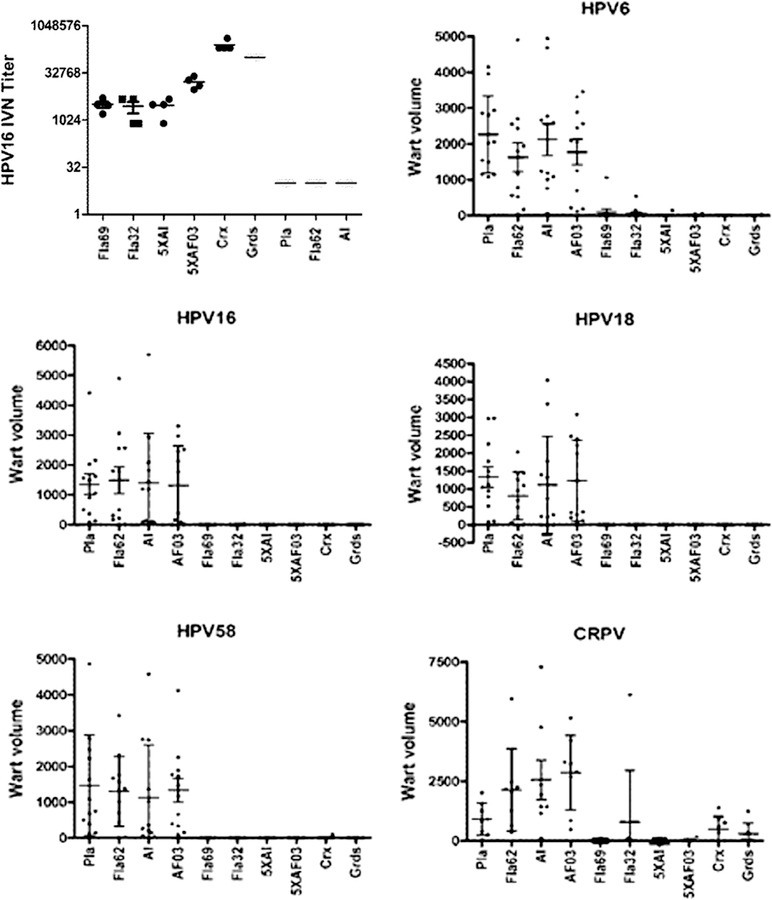
Fig. 5.
Impact of adjuvant on protection. Groups of 4 rabbits were immunized four times with placebo (Pla), 125 µg of flagellin only (Fla62), aluminum phosphate adjuvant only (Al), AF03 adjuvant only (AF03), or 125 µg Fla32, Fla69, or 125 µg α11–88×5 formulated in aluminum phosphate (5XAl), or with AF03 adjuvant (5XAF03), or full human doses of Cervarix or Gardasil on days 0, 21, 35 and 56. On day 77 serum was harvested and tested for HPV16 neutralizing antibody titer (top left panel). The rabbits were challenged on the skin with quazivirus of HPV types 6, 16, 18, 58 and CRPV. Graphs show wart volume readings 8 weeks post-challenge.