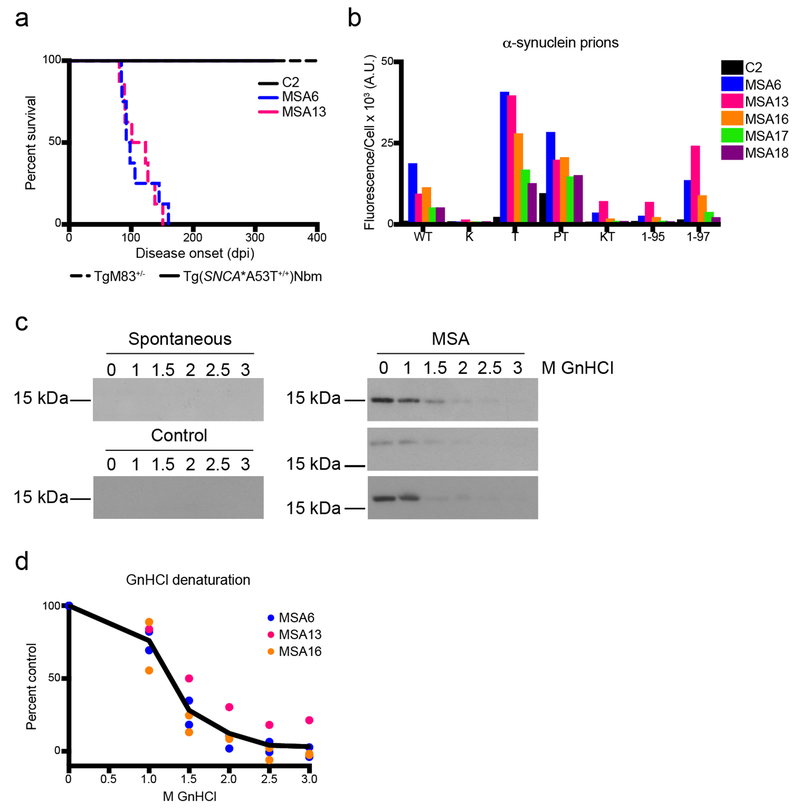
Fig. 1. MSA prions retain biological and biochemical properties after passaging in Tg(SNCA*A53T+/+) mice.
(a) Kaplan–Meier plot of disease onset in TgM83+/− (dotted lines) and Tg(SNCA*A53T+/+)Nbm mice (solid lines) inoculated with either control sample C2 or MSA patient samples MSA6 and MSA13. TgM83+/− mice inoculated with MSA6 and MSA13 developed neurological disease (incubation times in Online Resource, Table S2; data previously reported in [31]). Tg(SNCA*A53T+/+)Nbm did not develop overt motor signs by 330 dpi. (b–d) Brain homogenate from Tg(SNCA*A53T+/+)Nbm mice that propagated MSA prions, as well as mice inoculated with control sample C2, were assessed for (b) biological and (c, d) biochemical properties of MSA prions. (b) Alpha-synuclein prions were isolated from brain homogenates via PTA precipitation and were incubated with α-syn–YFP cells for 4 d. Homogenates from MSA-inoculated mice showed poor infectivity in cells expressing the E46K mutation alone or in combination with the A53T mutation, and MSA prions were not able to propagate well in cells truncated at residue 95. Homogenates from mice inoculated with sample C2 showed no infectivity. Cell lines express WT α-syn–YFP, single mutations [A30P (P), E46K (K), and A53T (T)], double mutations [A30P,A53T (PT) and E46K,A53T (KT)] or A53T truncated at residue 95 (1-95) or 97 (1-97). (c, d) Alpha-synuclein prions in MSA-inoculated mice were denatured using increasing concentrations of GnHCl. Representative blots using the EP1536Y primary antibody are shown in (c). No insoluble protein was detected in aged Tg(SNCA*A53T+/+)Nbm mice or animals inoculated with C2 homogenate.