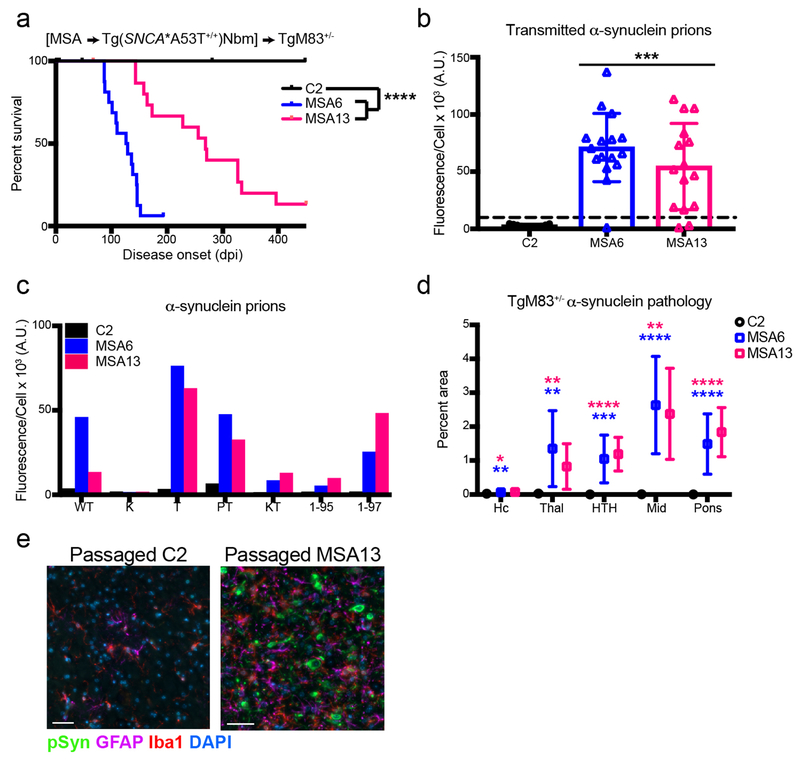
Fig. 7. MSA prions transmit disease to TgM83+/− mice after passaging in Tg(SNCA*A53T+/+)Nbm mice.
Brain homogenate from Tg(SNCA*A53T+/+)Nbm mice inoculated with either control (C2) or MSA (MSA6 or MSA13) patient samples was inoculated into TgM83+/− mice. (a) Mice inoculated with mouse-passaged MSA samples developed neurological disease, whereas mice inoculated with passaged C2 homogenate remained asymptomatic through 450 dpi. (b) Frozen half-brains from inoculated TgM83+/− mice were tested for MSA prions in the α-syn140*A53T–YFP cell assay (× 103 A.U.). Inoculation with passaged C2 homogenate did not induce α-synuclein prions (all samples below the dotted line), whereas mouse-passaged MSA samples induced α-synuclein prion formation. (c) Alpha-synuclein prions in TgM83+/− mice were analyzed for infectivity in additional α-syn–YFP cell lines. Samples infected cells expressing WT and mutant α-synuclein (A53T – T; A30P,A53T – PT; and A53T truncated at residue 97 – 1–97), but they did not infect cells expressing the E46K mutation alone (K) or in combination with the A53T mutation (KT). Additionally, they did not infect A53T-expressing cells truncated at residue 95 (1-95). (d, e) Fixed half-brains from the same mice were analyzed for phosphorylated α-synuclein neuropathology. Mice inoculated with the mouse-passaged C2 patient sample (n=11) did not develop α-synuclein aggregates, whereas inoculation of passaged MSA patient samples (MSA6: n=13; MSA13: n=9) induced widespread α-synuclein accumulation (green) and reactive astrocytes (GFAP; purple) and microglia (Iba1; red) in the hindbrain. Quantification of α-synuclein in the hippocampus (Hc), thalamus (Thal), hypothalamus (HTH), midbrain (Mid), and pons is shown in (d). Analysis was performed on one slide containing all brain regions from each animal. Representative images from the brainstem shown in (e). DAPI in blue. Scale bar, 50 μm. * = P < 0.05; ** = P < 0.01; *** = P < 0.001; **** = P < 0.0001.