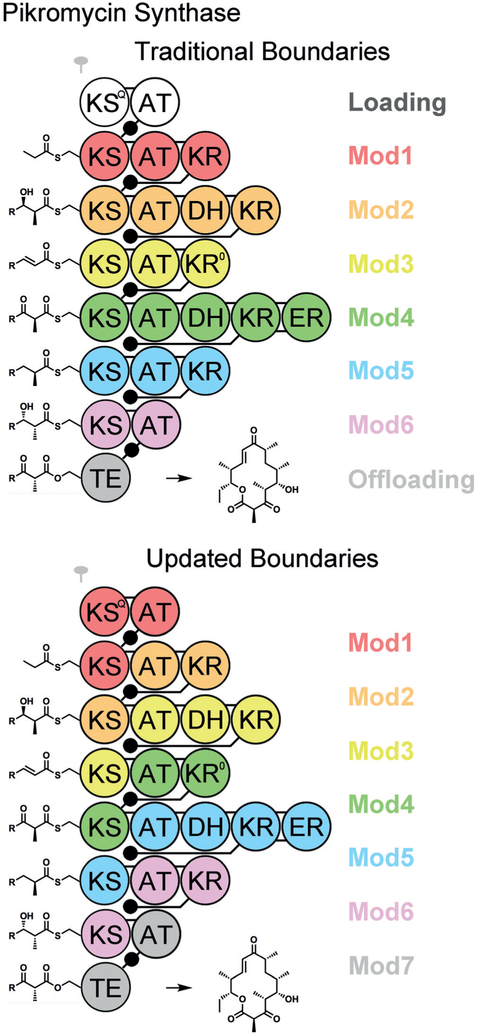
Figure 2.
The pikromycin synthase, color-coded with the traditional and updated module boundaries. The numbering of KS domains is not altered but the numbering of all other domains is increased by one. Employing the updated module boundaries, an ACP visits its own AT to acquire an extender unit, the KS of the previous module to collect the growing acyl chain through carbon–carbon bond formation, and its own processing enzymes before delivering the properly processed intermediate to its own KS. KSQ generates the priming propionyl group for the first KS through the decarboxylation of a methylmalonyl group, and belongs to the first module. The KS from module 4 selects between two intermediates interconverted by the epimerase KR0.