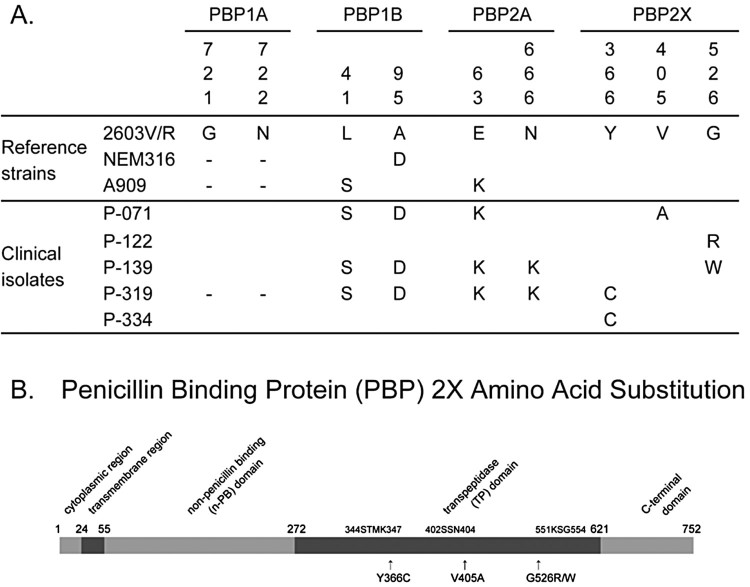
Figure 1.
(A) Amino acid sequences of penicillin binding proteins (PBPs) of control strains (2603V/R, NEM316, A909) and clinical isolates with reduced ceftibuten susceptibility recovered from pregnant women. The position of each amino acid is indicated above the sequence. “–” indicates an amino acid deletion. Amino acid substitutions found in PBP1A, 1B, and 2A of clinical isolates in the present study were previously found in penicillin-susceptible group B Streptococcus. We did not find any amino acid substitutions of PBP2B in clinical isolates. (B) PBP2X diagram and amino acid substitutions. The five GBS-RBS isolates in this investigation possessed single amino acid substitutions near the active-site motifs of the transpeptidase domain of PBP2X (Y366C, V405A, and G526R/W).