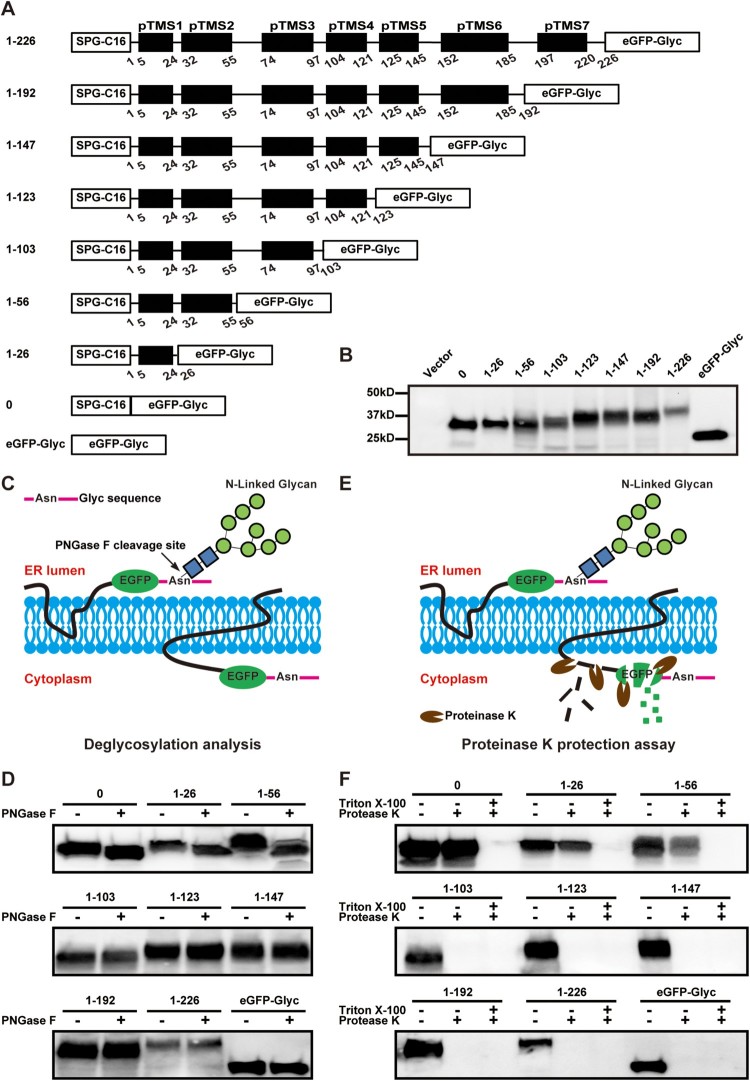
Figure 7.
Probing the membrane topology of ZIKV NS2A by in vitro deglycosylation assay and in vitro proteinase K protection assay. (A) Schematic representation of C-terminally truncated ZIKV NS2A proteins. The amino acid positions of each truncated NS2A are indicated. A ‘SPG-C16’ sequence was fused to the N terminus of NS2A to ensure correct translocation of NS2A on the ER membrane. An ‘eGFP-Glyc’ sequence was fused to the C terminus of NS2A to probe its location at cytosol or ER lumen. See text for details. (B) BHK-21 cells were transfected with the expression plasmids described in (A). At 24 h p.t., the expression levels of various NS2A truncates were examined by Western blot using an eGFP antibody. The amino acid positions are indicated for each NS2A truncates. The molecular masses of marker proteins are shown to the left of Western blot image. (C) Diagram of glycosylation of eGFP-Glyc when located at ER lumen (left). No glycosylation occurs when eGFP-Glyc is located at cytosol (right). The glycosylation residue Asn is indicated. (D) In vitro deglycosylation assay. Plasmid-transfected cells were treated with PNGase F or PBS according to the protocol of in vitro deglycosylation assay (see Materials and Methods). The samples were examined for glycosylation status by Western blot using an eGFP antibody. (E) Diagram of in vitro proteinase K protection assay. When eGFP is located at ER lumen, it is protected from proteinase K cleavage (left); when located in the cytosol, eGFP is degraded by proteinase K (right). (F) In vitro proteinase K protection assay. Cell lysates from plasmid-transfected BHK-21 cells were treated with proteinase K or PBS according to the protocol of in vitro proteinase K protection assay (see Materials and Methods). The samples were detected for eGFP by Western blot using an eGFP antibody. Triton X-100 treatment is indicated.