Antibodies to Plasmodium falciparum VAR2CSA, a critical antigen that mediates placental malaria, can be acquired outside of pregnancy. Here we show that exposure to Plasmodium vivax, particularly P. vivax Duffy binding protein, can give rise to functional antibodies that recognize VAR2CSA.
Keywords: Vivax, falciparum, pregnancy, PvDBP, VAR2CSA, immunity, placental malaria
Abstract
Background
In pregnancy, Plasmodium falciparum parasites express the surface antigen VAR2CSA, which mediates adherence of red blood cells to chondroitin sulfate A (CSA) in the placenta. VAR2CSA antibodies are generally acquired during infection in pregnancy and are associated with protection from placental malaria. We observed previously that men and children in Colombia also had antibodies to VAR2CSA, but the origin of these antibodies was unknown. Here, we tested whether infection with Plasmodium vivax is an alternative mechanism of acquisition of VAR2CSA antibodies.
Methods
We analyzed sera from nonpregnant Colombians and Brazilians exposed to P. vivax and monoclonal antibodies raised against P. vivax Duffy binding protein (PvDBP). Cross-reactivity to VAR2CSA was characterized by enzyme-linked immunosorbent assay, immunofluorescence assay, and flow cytometry, and antibodies were tested for inhibition of parasite binding to CSA.
Results
Over 50% of individuals had antibodies that recognized VAR2CSA. Affinity-purified PvDBP human antibodies and a PvDBP monoclonal antibody recognized VAR2CSA, showing that PvDBP can give rise to cross-reactive antibodies. Importantly, the monoclonal antibody inhibited parasite binding to CSA, which is the primary in vitro correlate of protection from placental malaria.
Conclusions
These data suggest that PvDBP induces antibodies that functionally recognize VAR2CSA, revealing a novel mechanism of cross-species immune recognition to falciparum malaria.
Malaria during pregnancy is responsible for 200000 infant deaths and 10000 maternal deaths annually [1]. Most deaths occur in sub-Saharan Africa and are caused by infection with Plasmodium falciparum. Morbidity and mortality from malaria during pregnancy are largely attributed to sequestration of parasites in the placenta, whereby infected red blood cells (RBCs) adhere to the syncytiotrophoblast, resulting in stillbirth, fetal growth restriction, low birth weight, and maternal anemia [2, 3].
Sequestration is a common mechanism used by P. falciparum to evade host immune defenses and clearance from the spleen. The surface antigen VAR2CSA plays a central role in the pathogenesis of placental malaria. Expression of this protein on the surface of infected RBCs mediates binding to chondroitin sulfate A (CSA) and parasite accumulation in the placental intervillous space [4]. Antibodies targeting VAR2CSA protect women from placental malaria and are associated with improved birth outcomes [5]. Protective antibodies typically arise in response to placental infection, and, as a result, antibodies are acquired in a parity-dependent manner. Primigravid women, who have lower levels of antibodies, have the greatest risk of complications from malaria during pregnancy, whereas multigravid women have acquired protective antibodies [6].
VAR2CSA is a member of the PfEMP1 family of proteins and contains 6 Duffy binding–like (DBL) domains and 3 interdomain regions [7]. It was discovered as an antigen that is upregulated in P. falciparum parasites from pregnant women [8] and in parasite strains selected in vitro for adhesion to CSA [4]. Although most studies report that antibodies to VAR2CSA are confined to pregnant populations, antibodies against the full-length protein or individual domains were reported in nonpregnant populations (ie, men and children) [9–13]. We previously observed a high frequency of antibodies in nonpregnant populations from Colombia [12]. Over 50% of men and children with acute infection with P. falciparum or Plasmodium vivax had antibodies that recognized the ID1-ID2, DBL3X, and DBL5ε domains of VAR2CSA. VAR2CSA-specific antibodies were also observed in pregnant women but were independent of parity. We further showed that antibodies from sera of pregnant and nonpregnant Colombians functionally inhibited parasite adhesion to CSA [12].
Neither the origin of these VAR2CSA cross-reactive antibodies nor their role in protecting against placental malaria is known. One hypothesis is that antibodies against VAR2CSA acquired outside of pregnancy could originate from exposure to P. vivax, which cocirculates with P. falciparum in this region of Colombia. While there is no direct homologue of VAR2CSA in P. vivax, 2 proteins (P. vivax Duffy binding protein [PvDBP] and erythrocyte binding protein 2 [EBP2]) contain DBL domains structurally similar to the 6 domains in VAR2CSA [14–16]. Little is known about the function of EBP2. PvDBP interacts with the Duffy antigen receptor for chemokines (DARC) on reticulocytes [17], and antibodies to PvDBP are observed in sera from patients exposed to P. vivax [18].
Here we demonstrate that men and children from Colombia and Brazil who were exposed to P. vivax have antibodies to full-length VAR2CSA. We show that cross-reactive antibodies can originate from exposure to PvDBP during infection and in mice following immunization with the DBL domain of PvDBP. We show that the mouse PvDBP monoclonal antibody recognizes the surface of VAR2CSA-expressing parasites and inhibits parasite binding to CSA in vitro, supporting a role for functional cross-recognition of P. vivax PvDBP antibodies against VAR2CSA.
METHODS
Ethical Considerations
Study approval was granted by the Health Research Ethics Board of the University of Alberta in Canada (approval Pro00041720); the Comité de Ética of Instituto de Investigaciones Médicas of Universidad de Antioquia in Colombia (approvals 009-2013, 002-2015, and 009-2016); the Ethics Committee of the Fundação Oswaldo Cruz, the Brazilian Ministry of Health, and the Ethical Committee of Research on Human Beings from the CPqRR/Fundação Oswaldo Cruz (reports 07/2009 and 26/2013; CAEE:50522115.7.0000.5091/05/2016), the Comité Consultatif de Déontologie et d’Ethique of the Research Institute for Development in France; the ethical committee of the Faculty of Health Science in Benin; and the Higher Degrees, Research, and Ethics Committee in Uganda (HDREC approval 368). All participants voluntarily provided written consent.
Study Region and Study Design
Samples from individuals in 2 Latin American countries (Colombia and Brazil) were included in this study (Table 1). Both symptomatic and asymptomatic men and children exposed to malaria were recruited in Colombia between 2013 and 2016, in the municipality of Puerto Libertador in the Department of Córdoba (7° 53′ 17″ N, 75° 40′ 18″ W; mean altitude, 66 m above sea level). The symptomatic population included patients who presented to the clinic with suspected malaria. Sera from the community were collected during a cross-sectional survey. Epidemiologic characteristics of this region are described elsewhere [19]. Briefly, the transmission intensity is low and stable, with no marked fluctuations in the number of malaria cases during the year; the entomological inoculation rate ranges from 3.5 to 4.8 infective bites per person per year [20]. During 2013–2016, the annual parasitic index (calculated as the number of malaria cases/1000 inhabitants) in our study region ranged from 1.2 to 2.7 for P. vivax and from 0.1 to 0.7 for P. falciparum (Instituto Nacional de Salud, Colombia). Fifty sera from residents of Medellín, where there is no malaria, were included as unexposed controls.
Table 1.
Study Population
| Country, Town | Malaria Status | Participants, No. | Sex, M:F |
|---|---|---|---|
| Colombia | |||
| Puerto Libertador | Exposed to P. falciparum and P. vivax | 156a | 154:2b |
| Medellín | No exposure | 50 | 25:25 |
| Brazil | |||
| Rio Pardo | Exposed to P. vivax | 41 | 41:0 |
| Belo Horizonte | No exposure | 10 | 4:6 |
| Canada | |||
| Edmonton | No exposure | 21 | 5:16 |
Abbreviations: P. falciparum, Plasmodium falciparum; P. vivax, Plasmodium vivax.
aIncludes 9 men and children whose sera were used for affinity purification.
bFemales were ≤12 years old.
In Brazil, malaria-exposed participants were recruited in the agricultural settlement of Rio Pardo (1o 46′ S–1o 54′ S, 60o 22′ W–60o 10′ W), Presidente Figueiredo municipality, northeast Amazonas State in the Brazilian Amazon area. The study site and malaria transmission patterns were described elsewhere [21]. A population-based open cohort study was initiated in November 2008, where 541 inhabitants were enrolled at baseline, and after 6 and 12 months, 2 similar cross-sectional surveys were performed. The annual parasitic index in 2008–2009 ranged from 199.0 to 307.6 for P. vivax and from 6.5 to 26.2 for P. falciparum [21, 22]. In addition, plasma samples from malaria-unexposed Brazilian adults were collected in the region of Belo Horizonte, Minas Gerais State.
Positive controls included pooled sera from pregnant women from Benin and Uganda who participated in other studies. Additional unexposed controls included sera from 21 Canadian residents (5 men and 16 women), with no history of travel to malaria-endemic countries. These samples were collected in a previous study [12].
Sample Collection
Blood samples from Colombian participants (<5 mL) were collected by venipuncture. Thick and thin blood films were prepared, stained with Giemsa, and diagnosed by microscopy. Total nucleic acid was extracted from red blood cell pellets, and malaria diagnosis was performed by reverse transcription quantitative polymerase chain reaction (qPCR) analysis as described elsewhere [23]. Whole-blood and plasma specimens from Brazilian participants were collected as described previously [24], and malaria diagnosis was based on qPCR findings [25].
Enzyme-Linked Immunosorbent Assay (ELISA)
The specific levels of antibodies (immunoglobulin G [IgG]) were measured in serum samples, using ELISA as described elsewhere [12]. P. falciparum MSP1 (PfMSP1) and P. vivax MSP1 (PvMSP1) were purchased commercially (CTK Biotech, San Diego, CA). VAR2CSA DBL domains ID1-ID2 and DBL4ε (FCR3 allele) and IT4var07 CIDRα1.4 were produced in Escherichia coli as described elsewhere [26]. DBL domains DBL3X and DBL5ε, and full-length VAR2CSA (FCR3 allele) produced as described previously [27]. The DBPII region of the PvDBP DBL domain was expressed and purified as previously described [28]. Plates were coated with 0.5 μg/mL of each antigen. ODs were converted to arbitrary units (AU) [29] relative to a pool of sera from multigravid women from Benin that was run on each plate, according to the formula: AU = [(ODtest sera – background)/(ODBenin pool – background)]*100. Background was the OD of the antigen with secondary antibody alone. Cutoffs for each antigen were determined on the basis of the mean OD of individual Canadian sera (19–21 sera) plus 2 SDs and converted to AU against the Beninese multigravid control on the same plate. Two mouse monoclonal antibodies (mAbs), 3D10 and 2D10 (both IgG1), were developed following immunization with the DBPII domain of PvDBP [28] and were compared to a commercial isotype control (Invitrogen, Waltham, MA).
Affinity Purification
Recombinant PvDBP (DBPII region; 3 mg) was loaded onto a HiTrap NHS-Activated HP column (GE Healthcare Life Sciences). Sera from 9 Colombian men and children with antibodies to PvDBP were pooled, loaded onto the column, and eluted. Total IgG was purified further using a HiTrap Protein G HP column (GE Healthcare Life Sciences) and quantified using a Nanodrop. The elution fractions containing IgG were concentrated, and the buffer was exchanged with 1× phosphate-buffered saline (PBS), using an Amicon Ultra-4 centrifugal filter (Merck Millipore).
P. falciparum Culture
P. falciparum CS2 strain (FCR3 allele of VAR2CSA), NF54-CSA (3D7 allele of VAR2CSA), and a placental isolate [30] were cultured in vitro, as described previously [31]. Parasites were selected on CSA (C9819; Sigma-Aldrich) to enrich for parasites expressing VAR2CSA. Mature parasites were magnetically purified using the VarioMACS according to the manufacturer’s instructions (Miltenyl Biotec).
Immunofluorescence Assay (IFA)
Thin smears of magnetically purified P. falciparum CS2–infected RBCs were fixed with methanol, washed in 1× PBS, blocked in 3% BSA/1× PBS for 1 hour at room temperature, and placed in a humidified chamber at 4°C overnight with primary antibody at a ratio of 1:100 in 1% BSA/1× PBS. Slides were washed, and secondary antibody (Alexa555 goat anti-mouse or Alexa647 goat anti-rabbit; Molecular Probes) was diluted 1:500 in 1% BSA/1× PBS and incubated for 2 hours at room temperature. Prolong Gold Antifade reagent containing DAPI (Molecular Probes) was applied, and slides were mounted with a number 1.5 cover glass. Image data were collected using a Leica SP5 laser scanning confocal microscope equipped with a 100×/1.44 oil-immersion lens. A Gaussian filter was applied with a kernel size of 5, using Leica LASAF software. Figures were compiled using ImageJ.
Flow Cytometry
Mature parasites (1 × 105 cells) from P. falciparum strains CS2 and NF54-CSA and a placental isolate were incubated for 1 hour at 4°C with the 3D10 mAb or isotype control (143 µg/mL). Mouse antibodies were preabsorbed on uninfected RBCs and detected with AlexaFluor 647–conjugated goat anti-mouse IgG (dilution, 1:500; Life Technologies). Rabbit anti-VAR2CSA or normal rabbit serum was preabsorbed on uninfected RBCs, incubated with infected RBCs at a ratio of 1:40, and detected with AlexaFluor 647–conjugated goat anti-rabbit IgG (dilution, 1:500; Life Technologies). Parasite DNA was stained with 5 µg/mL of DAPI. Uninfected RBCs were excluded on the basis of DAPI staining. DAPI-positive infected RBCs (>11000 cells) were quantified by flow cytometry (Fortessa X20), and data were analyzed with FlowJo, version 7.6 (TreeStar, Ashland, OR). For fluorescent flow microscopy, CS2-infected RBCs were stained as described above. Images of individual infected RBCs were captured using the ImageStreamX Mark II Imaging Flow Cytometer (Inspire v6.2; Amnis, Seattle, WA) and analyzed using Ideas software (v6.2; Amnis).
Inhibition of Binding Assay (IBA)
The ability of antibodies to interfere with CSA adhesion of infected RBCs was assessed by a modified static IBA protocol [12]. Total IgG was purified from sera of Colombian men and children positive for antibodies to PvMSP1 and VAR2CSA but negative for antibodies to PfMSP1 (sera from Figure 1B that had an AU for VAR2CSA above the cutoff), using a HiTrap Protein G HP column (GE Healthcare Life Sciences) and quantified using a Nanodrop. The control total IgG was purified from a pool of sera from Colombian men and women living in Medellín. IgGs were tested in the IBA at a concentration of 4 mg/mL. The mouse PvDBP monoclonal 3D10 and the IgG1 isotype control were tested in the IBA at a concentration of 100 μg/mL. Following incubation with antibodies and infected RBCs, the entire CSA spot was imaged with a 4× objective, using an EVOS FL Auto microscope (Invitrogen) equipped with a 4×/0.13 phase objective lens, and the number of bound infected RBCs on each spot was quantified using ImageJ. All experiments included replicates across multiple plates and strains and were performed at least twice. The percentage inhibition was calculated as the number of parasites bound per spot after incubation with the test sera or IgG, divided by findings for the control, with the sCSA counts considered indicative of 100% maximum inhibition.
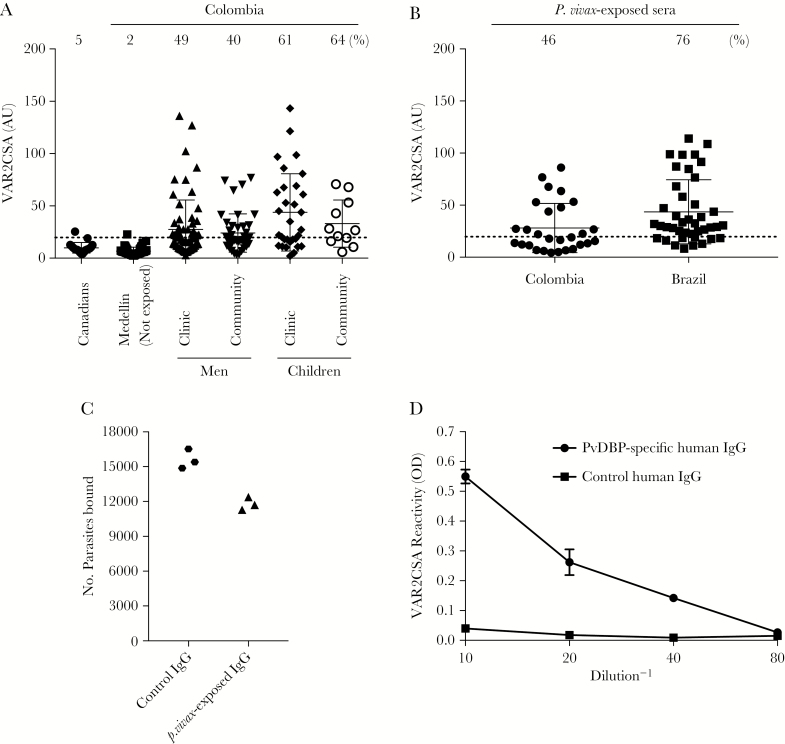
Figure 1.
Human antibodies to Plasmodium vivax Duffy binding protein (PvDBP) recognize the Plasmodium falciparum surface antigen VAR2CSA. A, Sera from different populations in Colombia were tested for reactivity to full-length VAR2CSA (based on detection of the FCR3 allele) by enzyme-linked immunosorbent assay (ELISA; dilution, 1:1000). Values are expressed as mean AUs (±SD) relative to a positive control on each plate. A cutoff (stippled line) was determined for each antigen, based on the mean ODs (+2 SDs) for individual sera from the Canadian population. The percentage of samples with an AU above the cutoff is indicated for each population. AUs of the sera from the malaria-exposed groups in the clinic and community were significantly different from that for the unexposed group from Medellín, using a Dunn multiple comparisons test (P < .0001, for all comparisons between groups). B, VAR2CSA reactivity in sera positive against P. vivax MSP1 (PvMSP1) and negative against P. falciparum MSP1 (PfMSP1). Samples were collected from individuals in Colombia and Brazil and were analyzed against VAR2CSA as described in panel A. C, Colombian sera from panel B that were seropositive against VAR2CSA and with P. vivax exposure were pooled, and immunoglobulin G (IgG) was purified. Total IgG was tested in the inhibition of binding assay against a placental isolate that was adapted to in vitro culture. Total IgG was purified from the pooled sera of unexposed Colombians as control IgG. Results are expressed as the number of parasites bound to chondroitin sulfate A (CSA) from triplicates of a representative experiment. D, PvDBP antibodies underwent affinity purification from a pool of sera from Colombian men and children previously exposed to P. vivax and titrated against VAR2CSA by ELISA, with an initial IgG concentration of 12 μg/mL (1:10 dilution on the x-axis). IgG from unexposed Colombians served as the control. Data are mean ODs (±SD).
Statistical Analyses
Data were plotted using Prism software (version 7; GraphPad). A D’Agostino-Pearson test was used to determine whether data sets for the serologic data (Figure 1A) followed a normal distribution. Comparisons of VAR2CSA AU data were made using a Kruskal-Wallis test and Dunn multiple comparisons test.
RESULTS
Populations Exposed to P. vivax Have VAR2CSA Antibodies
We first tested whether nonpregnant populations living in a malaria-endemic region of Colombia have VAR2CSA antibodies (Figure 1A; Table 2). Negative controls included Canadians and nonexposed Colombians living in Medellín (all were confirmed to be negative for malarial parasite infection by qPCR). In the negative controls, few had antibodies to VAR2CSA (Figure 1A). In contrast, 50 of 94 men and children who presented to the clinic in Puerto Libertador had VAR2CSA antibodies (49% and 61%, respectively; Figure 1A). Fifty-three asymptomatic men and children from the community (all of whom were PCR negative; Table 2) also had VAR2CSA antibodies (40% and 64%, respectively; Figure 1A). These results are consistent with our published data that Colombian men and children had antibodies recognizing the recombinant VAR2CSA domains ID1-ID2, DBL3X, and DBL5ε [12]. Given that the full-length protein has a higher affinity for CSA than the individual domains [32], we focused on the cross-reactivity to the entire folded protein, rather than to the individual domains.
Table 2.
Nonpregnant Populations from Puerto Libertador, Colombia, Tested for Serum Reactivity to the Plasmodium falciparum Surface Antigen VAR2CSA
| Group, Recruitment Site | Participants, No. | Species Detected (No.) |
|---|---|---|
| % positive | ||
| Men (n = 105) | ||
| Clinic | 63 | … |
| Positive | 30 | P. vivax (22), P. falciparum (8) |
| Negative | 33 | None |
| Community | 42 | … |
| Negative | 42 | None |
| Childrena (n = 42) | ||
| Clinic | 31 | … |
| Positive | 15 | P. vivax (9), P. falciparum (6) |
| Negative | 16 | None |
| Community | 11 | … |
| Negative | 11 | None |
Abbreviations: P. vivax, Plasmodium vivax; qPCR, quantitative polymerase chain reaction.
aIncludes 2 girls ≤12 years of age
We hypothesized that VAR2CSA antibodies acquired outside of pregnancy arose from exposure to P. vivax. To determine whether Colombian men and children were exposed to different species of Plasmodium, serologic testing was performed using PfMSP1 and PvMSP1. Negative PfMSP1 serologic findings suggest a lack of recent exposure to P. falciparum, based on the long antibody half-life (7.6 years in settings of similarly low transmission) [33] and detection of PfMSP1 antibodies in 80% of individuals recently infected with P. falciparum [34]. We identified 28 sera reactive only to PvMSP1. Forty-six percent of sera had antibodies that recognized VAR2CSA (Figure 1B). While a lack of antibodies to PfMSP1 does not definitively exclude prior exposure to P. falciparum, we subsequently identified a population in the Brazilian Amazon with no documented infection with P. falciparum during the 5 years before sample collection, according to the Brazil Epidemiological Surveillance Information System. After screening by serologic analysis, sera from 41 men and children were positive for antibodies to PvMSP1 and negative for antibodies against PfMSP1. Of these, 31 (76%) had antibodies that recognized VAR2CSA (Figure 1B), consistent with the high frequency of cross-reactive antibodies in our Colombian population.
To test whether VAR2CSA antibodies in the P. vivax-exposed populations are functional, total IgG was purified from the Colombian sera that were positive for VAR2CSA, pooled, and tested in triplicate in the IBA, in which infected RBCs were incubated with antibody prior to being added to CSA adsorbed to plastic. These IgGs blocked binding to CSA of a P. falciparum placental isolate (adapted to in vitro culture) by 24% relative to total IgG purified from a pool of sera from Medellín (Figure 1C). This experiment was repeated with the CS2 strain with both purified IgG and total serum, with similar results. The mean percentage inhibition (±standard error of the mean [SEM]) across all experiments and strains (5 independent experiments) was 22.3% ± 3.9%.
PvDBP and VAR2CSA Share Common Epitopes
DBL domains in VAR2CSA and PvDBP share structural similarities [16] and 16%–21% sequence identity (Supplementary Figure 1). We therefore asked whether PvDBP antibodies in P. vivax–exposed individuals would cross-react with VAR2CSA, thus identifying a potential source for these antibodies. We performed affinity purification of PvDBP IgG from pooled sera from Colombian men and children and found that PvDBP-specific human antibodies reacted with VAR2CSA, with an end point titer of 3 µg/mL (Figure 1D). Total IgG purified from sera of Medellín residents was used as a negative control. These data suggest that exposure to PvDBP following P. vivax infection is one possible source of antibodies that recognize VAR2CSA.
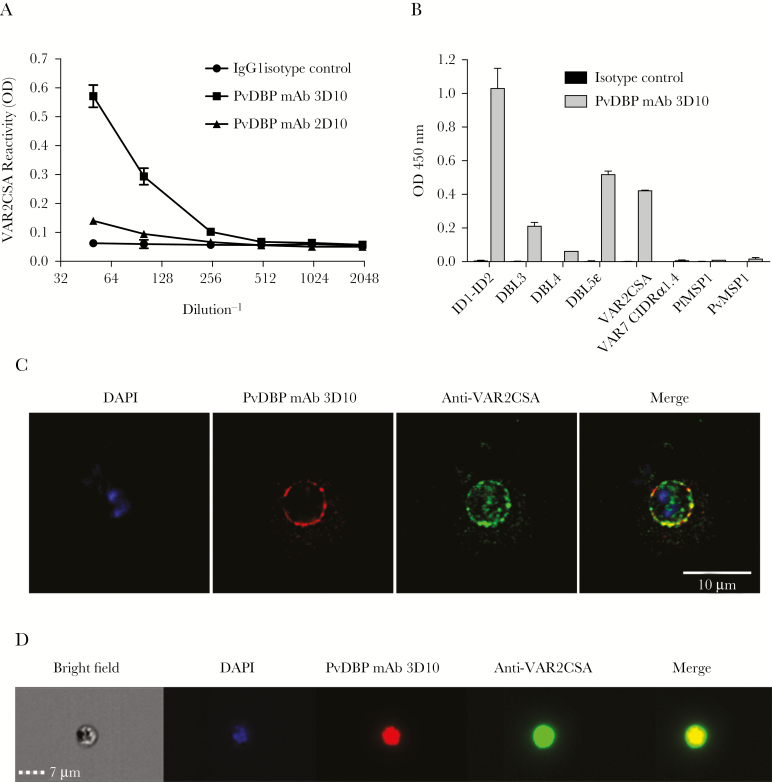
To determine whether immunization with PvDBP can generate cross-reactive antibodies, we took advantage of 2 monoclonal antibodies, 3D10 and 2D10 (both IgG1), that developed following immunization with the DBPII domain of PvDBP [28]. We tested their reactivity to VAR2CSA and observed strong recognition with 3D10, with an end point titer of 0.86 µg/mL, but much weaker recognition with 2D10 (Figure 2A). The 3D10 mAb also recognized individual recombinant DBL domains from VAR2CSA, with the strongest reactivity against DBL5ε and ID1-ID2 (Figure 2B). This indicated that there was at least 1 epitope of PvDBP that was shared with different domains of VAR2CSA.
Figure 2.
A Plasmodium vivax Duffy binding protein (PvDBP) mouse monoclonal antibody recognizes the Plasmodium falciparum surface antigen VAR2CSA. A, Titration of 2 PvDBP mouse monoclonal antibodies (mAbs; 3D10 and 2D10) and control immunoglobulin G1 (IgG1) against full-length VAR2CSA (FCR3) by enzyme-linked immunosorbent assay (ELISA). The concentration of the first dilution (1:50 on the x-axis) of each antibody was 8.6 μg/mL. Data are mean ODs (±SD). B, PvDBP mAb 3D10 and the isotype control (8.6 μg/mL for both) were tested against various recombinant Duffy binding–like (DBL) domains from VAR2CSA (ID1-ID2, DBL3X, DBL4ε, and DBL5ε), full-length VAR2CSA, IT4var07 CIDRα1.4, against P. falciparum MSP1 (PvMSP1), and against P. vivax MSP1 (PvMSP1) by ELISA, all coated at 0.5 μg/mL. C, red blood cells (RBCs) infected with mature P. falciparum strain CS2 parasites were fixed and costained with DAPI (blue), PvDBP mAb 3D10 (red), and a rabbit polyclonal antibody to VAR2CSA (green). Controls are shown in Supplementary Figure 2. D, Live cell images of a representative CS2-infected RBC (under bright field and with DAPI costaining) that shows costaining of PvDBP mAb 3D10 (red) and anti-VAR2CSA antibody (green).
Based on the cross-reactivity of 3D10 to VAR2CSA, we used IFA to test whether it recognized the protein expressed on the surface of infected RBCs. Mature forms of P. falciparum CS2–infected RBCs that expressed VAR2CSA were fixed and stained with 3D10 and visualized by immunofluorescence (Figure 2C). We observed staining at or near the surface of infected RBCs. The pattern of recognition was similar to that seen when infected RBCs were costained with rabbit anti-VAR2CSA antibodies. Neither antibody stained uninfected red cells. Normal mouse serum and normal rabbit serum did not stain CS2-infected RBCs (Supplementary Figure 2). 3D10 also recognized live infected RBCs (Figure 2D), as observed by live cell imaging (Image Stream), with 3D10 and VAR2CSA antibodies co-localizing to the cell surface.
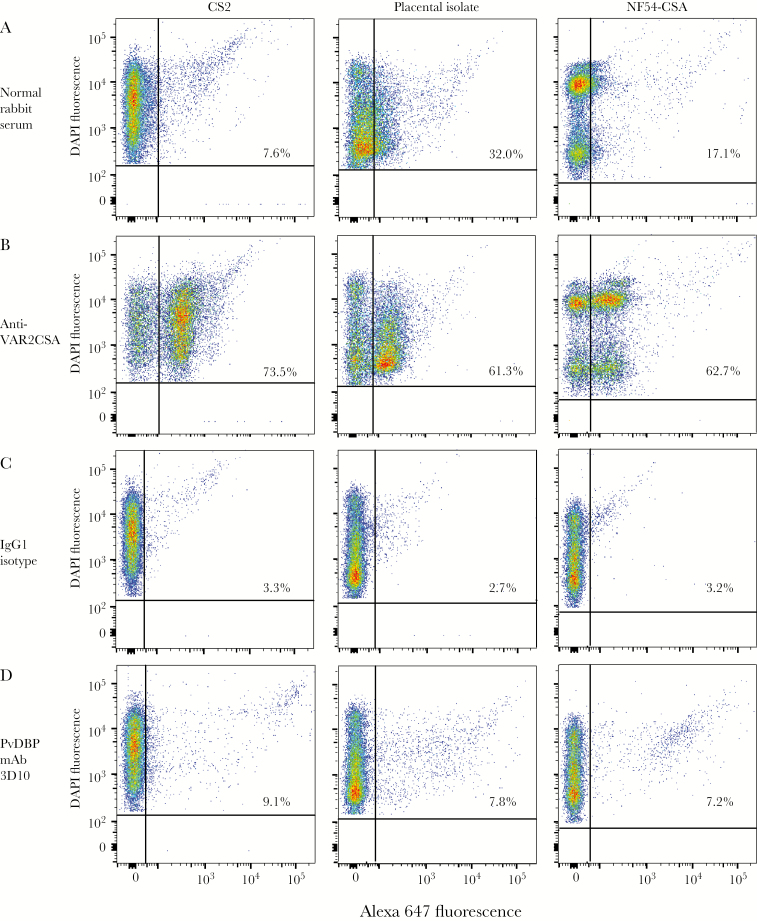
Next, we quantified the population of cells recognized by 3D10, using flow cytometry (Figure 3). All strains were selected on CSA, and >60% of infected RBCs expressed VAR2CSA on the cell surface (Figure 3A and 3B). We observed that 9.1% of RBCs infected with CS2 parasites stained with 3D10, compared with 3.3% for the IgG1 isotype control (Figure 3C and 3D). While the overall percentage was low, the proportion of stained cells was higher with 3D10 as compared to the isotype control, and we observed high staining of individual cells recognized by the antibody. 3D10 recognized populations of trophozoites and schizonts, whereas only 0.22% of RBCs enriched for rings were recognized (data not shown). 3D10 recognized a similar population of infected RBCs from a placental isolate and NF54-CSA (Figure 3C and 3D), suggesting that the antibody to PvDBP cross-reacts with multiple alleles of VAR2CSA. The small proportion of live stained cells as compared to the fixed cells could indicate that the affinity of the cross-reactive antibodies is low and that the antibodies are sensitive to the washing during the flow procedure. Alternatively, either the expression of the epitope recognized by 3D10 on the surface of infected RBCs may be transient or the epitope is partially embedded within the plasma membrane.
Figure 3.
Plasmodium vivax Duffy binding protein (PvDBP) monoclonal antibody (mAb) 3D10 recognizes live Plasmodium falciparum strain CS2–infected red blood cells (RBCs). CS2, a placental isolate, and NF54–chondroitin sulfate A (CSA) infected RBCs were analyzed by flow cytometry. A and B, To verify the expression of VAR2CSA, infected RBCs were stained with normal rabbit serum (A) and a polyclonal anti-VAR2CSA rabbit antibody (B), both at a 1:40 dilution. C and D, All 3 strains were stained with the immunoglobulin G1 (IgG1) isotype control (C) and PvDBP 3D10 mAb (D), both at 143 μg/mL. The percentage of infected RBCs recognized by the antibody is indicated on each plot.
Inhibition of Infected RBC Binding to CSA by PvDBP mAb 3D10
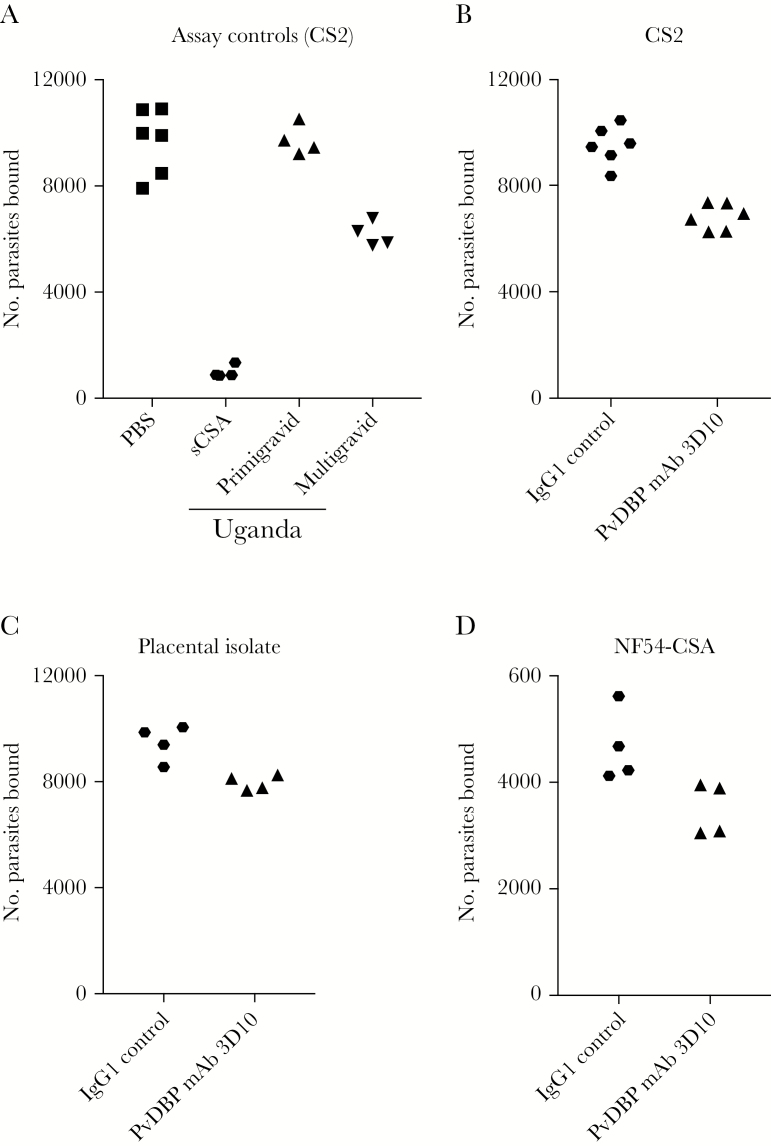
To test the functional activity of 3D10, we asked whether it could block infected RBC binding to CSA (Figure 4). Three parasite strains were tested that expressed high surface levels of VAR2CSA. Sera from primigravid and multigravid women from Uganda and soluble CSA were used as controls to measure inhibition of binding (Figure 4A). 3D10 inhibited binding of CS2-infected RBCs to CSA by 32% relative to the IgG1 isotype control (Figure 4B). Binding inhibition, although partial, was also observed with the placental isolate and NF54-CSA (Figure 4C and 4D), suggesting that the epitope recognized by 3D10 may be conserved between falciparum strains. The mean percentage inhibition (±SEM) across all experiments and strains (8 independent experiments) was 18.1% ± 3.0%.
Figure 4.
Plasmodium vivax Duffy binding protein (PvDBP) monoclonal antibody (mAb) 3D10 blocks adhesion of infected red blood cells (RBCs) to chondroitin sulfate A (CSA). A, Controls for the inhibition of binding assay included Plasmodium falciparum strain–CS2 infected RBCs incubated with phosphate-buffered saline (PBS) alone, soluble CSA (sCSA), and sera from primigravid and multigravid women from Uganda. B–D, PvDBP mAb 3D10 was tested for inhibition of CS2 (B), a placental isolate (C), and NF54-CSA–infected RBC binding to CSA (D). Results are expressed as the number of parasites bound to CSA from replicates of a representative experiment. IgG1, immunoglobulin G1.
DISCUSSION
This is the first demonstration of cross-species functional immune recognition of proteins from 2 divergent species of Plasmodia that are involved in different virulence pathways. Antibodies against the DBL domain from PvDBP recognized DBL domains of P. falciparum VAR2CSA, which may contribute to cross-species immunity. We further showed that a PvDBP cross-reactive epitope is expressed on the surface of P. falciparum–infected RBCs, where it can sensitize the parasite to antibody-mediated inhibition of binding to CSA—the hallmark correlate of immunity to placental malaria. These findings are consistent with our previous findings that serum antibodies from Colombian men, women, and children (with exposure to both P. falciparum and P. vivax) could inhibit binding of P. falciparum infected RBCs to CSA [12]. Here, we identified one source of these antibodies: exposure to PvDBP. Recently, PvDBP antibodies were associated with increased infant birth weight in a study from countries where both P. vivax and P. falciparum are prevalent, including Colombia and Brazil [35]. We speculate that the increased birth weight in some infants is in response to cross-species protection against P. falciparum, leading to better pregnancy outcomes. This idea remains to be investigated in future studies.
Our findings are consistent with reports of broad antibody cross-reactivity between P. falciparum or P. vivax outside of pregnancy [36–39] and several examples of antigen-specific reactivity between these 2 species. Sera from P. vivax–exposed individuals from Brazil recognized peptides from PfCLAG9, and conversely, antibodies raised against these peptides in mice recognized P. vivax infected RBCs by IFA [40]. Cross-reactivity between orthologous proteins was reported for PfMSP5 and PvMSP5 [41], as well as PfAMA-1 and PvAMA-1 [42], and recently, cross-boosting was demonstrated between Pvs48/45 and Pfs48/45 in mice [43]. We provide the first evidence of cross-reactivity between nonorthologous proteins that are involved in different biological pathways: cytoadherence and parasite invasion. Both pathways are essential for parasite survival during blood-stage parasite infection, which may have led to evolutionary conservation of specific epitopes in this protein family. Although the mechanism is not yet known, it is possible that binding of 3D10 to VAR2CSA blocks infected RBC adhesion to CSA by steric hindrance. Based on our flow data, the target epitopes in VAR2CSA may be cryptic or exposed transiently, resulting in only a subset of infected RBCs recognized by 3D10. However, this small proportion of cells was stained very strongly by the antibody, which could account for the partial inhibition of adhesion to CSA that we observed in the IBA.
Cross-reactivity among DBL domains in P. falciparum and P. vivax may extend beyond the ones characterized here. It will be important to map the target cross-reactive epitopes within all 6 domains of VAR2CSA and even other DBL proteins from P. falciparum that play a role in cytoadherence or invasion. It is also possible that the DBL domain of EBP2 can induce antibodies that recognize VAR2CSA or other DBL proteins from P. falciparum. Given their structural similarity [15, 44], PvDBP is likely not the sole determinant of cross-reactivity to VAR2CSA.
In conclusion, we discovered a novel mechanism that may lead to natural immunity to P. falciparum through cross-species recognition of a structurally related DBL domain from P. vivax. The specific epitopes in PvDBP that induce functional antibodies and inhibit parasite adhesion to CSA may be exploited as an approach to a vaccine against pregnancy-associated malaria and complement current vaccines in development that are based on domains of VAR2CSA.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the study volunteers and field staff, for their participation in this work; Aja Rieger, for assistance with flow cytometry; and Louise Turner, for sharing the IT4var07 CIDRα1.4 recombinant protein.
Financial support. This work was supported by the Canadian Institutes of Health Research (open operating grant RES0016099 to S. K. Y. and Canada Graduate Scholarship to C. J. M., the Natural Sciences and Engineering Research Council of Canada (discovery grant RGPIN-2017–04179 to S. K. Y. and Postgraduate Scholarship-Doctoral to C. J. M.)), the Li Ka Shing Institute of Virology (translational research grant to S. K. Y.), Grand Challenges Canada (Stars in Global Health award to S. G.), the Universidad de Antioquia (Estrategia de Sostenibilidad 2014–2015 to A. M. and E. A.), the Natural Sciences and Engineering Research Council of Canada CREATE Host-Parasite Interactions Network (scholarship to A. M.), COLCIENCIAS (support to A. M.), the University of Alberta (research exchange scholarship to E. M.), the Australian NHMRC (support to M. F. G.), Alberta Innovates Health Solutions (visiting scholar award to M. F. G.), and the Intramural Research Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health (support to J. Y. D., D. L. N., and P. E. D.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: International Conference on Plasmodium Vivax Research, Manaus, Brazil, June 2017; Annual Meeting of the American Society of Tropical Medicine and Hygiene, Baltimore, Maryland, November 2017.
References
- 1. The contribution of malaria control to maternal and newborn health. In: Malaria in Pregnancy Consortium, ed. Progress & impact series. Geneva: World Health Organization on behalf of the Roll Back Malaria Partnership Secretariat, 2014. [Google Scholar]
- 2. Duffy PE. Plasmodium in the placenta: parasites, parity, protection, prevention and possibly preeclampsia. Parasitology 2007; 134:1877–81. [DOI] [PubMed] [Google Scholar]
- 3. Desai M, ter Kuile FO, Nosten F, et al. . Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 2007; 7:93–104. [DOI] [PubMed] [Google Scholar]
- 4. Salanti A, Dahlbäck M, Turner L, et al. . Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J Exp Med 2004; 200:1197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ataíde R, Mayor A, Rogerson SJ. Malaria, primigravidae, and antibodies: knowledge gained and future perspectives. Trends Parasitol 2014; 30:85–94. [DOI] [PubMed] [Google Scholar]
- 6. Duffy PE, Fried M. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect Immun 2003; 71:6620–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andersen P, Nielsen MA, Resende M, et al. . Structural insight into epitopes in the pregnancy-associated malaria protein VAR2CSA. PLoS Pathog 2008; 4:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salanti A, Staalsoe T, Lavstsen T, et al. . Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol Microbiol 2003; 49:179–91. [DOI] [PubMed] [Google Scholar]
- 9. Babakhanyan A, Leke RG, Salanti A, et al. . The antibody response of pregnant Cameroonian women to VAR2CSA ID1-ID2a, a small recombinant protein containing the CSA-binding site. PLoS One 2014; 9:e88173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beeson JG, Ndungu F, Persson KE, et al. . Antibodies among men and children to placental-binding Plasmodium falciparum-infected erythrocytes that express var2csa. Am J Trop Med Hyg 2007; 77:22–8. [PubMed] [Google Scholar]
- 11. Fodjo BA, Atemnkeng N, Esemu L, et al. . Antibody responses to the full-length VAR2CSA and its DBL domains in Cameroonian children and teenagers. Malar J 2016; 15:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gnidehou S, Doritchamou J, Arango EM, et al. . Functional antibodies against VAR2CSA in nonpregnant populations from colombia exposed to Plasmodium falciparum and Plasmodium vivax. Infect Immun 2014; 82:2565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oleinikov AV, Voronkova VV, Frye IT, et al. . A plasma survey using 38 PfEMP1 domains reveals frequent recognition of the Plasmodium falciparum antigen VAR2CSA among young Tanzanian children. PLoS One 2012; 7:e31011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adams JH, Sim BK, Dolan SA, Fang X, Kaslow DC, Miller LH. A family of erythrocyte binding proteins of malaria parasites. Proc Natl Acad Sci USA 1992; 89:7085–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hester J, Chan ER, Menard D, et al. . De novo assembly of a field isolate genome reveals novel Plasmodium vivax erythrocyte invasion genes. PLoS Negl Trop Dis 2013; 7:e2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Howell DP, Samudrala R, Smith JD. Disguising itself–insights into Plasmodium falciparum binding and immune evasion from the DBL crystal structure. Mol Biochem Parasitol 2006; 148:1–9. [DOI] [PubMed] [Google Scholar]
- 17. Choe H, Moore MJ, Owens CM, et al. . Sulphated tyrosines mediate association of chemokines and Plasmodium vivax Duffy binding protein with the Duffy antigen/receptor for chemokines (DARC). Mol Microbiol 2005; 55:1413–22. [DOI] [PubMed] [Google Scholar]
- 18. Tran TM, Oliveira-Ferreira J, Moreno A, et al. . Comparison of IgG reactivities to Plasmodium vivax merozoite invasion antigens in a Brazilian Amazon population. Am J Trop Med Hyg 2005; 73:244–55. [PubMed] [Google Scholar]
- 19. Rodríguez JC, Uribe GÁ, Araújo RM, Narváez PC, Valencia SH. Epidemiology and control of malaria in Colombia. Mem Inst Oswaldo Cruz 2011; 106(Suppl 1):114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Naranjo-Diaz N, Rosero DA, Rua-Uribe G, Luckhart S, Correa MM. Abundance, behavior and entomological inoculation rates of anthropophilic anophelines from a primary Colombian malaria endemic area. Parasit Vectors 2013; 6:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kano FS, Sanchez BA, Sousa TN, et al. . Plasmodium vivax Duffy binding protein: baseline antibody responses and parasite polymorphisms in a well-consolidated settlement of the Amazon region. Trop Med Int Health 2012; 17:989–1000. [DOI] [PubMed] [Google Scholar]
- 22. Kano FS, Souza-Silva FA, Torres LM, et al. . The presence, persistence and functional properties of Plasmodium vivax Duffy binding protein II antibodies are influenced by HLA class II allelic variants. PLoS Negl Trop Dis 2016; 10:e0005177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gavina K, Arango E, Larrotta CA, Maestre A, Yanow SK. A sensitive species-specific reverse transcription real-time PCR method for detection of Plasmodium falciparum and Plasmodium vivax. Parasite Epidemiol Control 2017; 2:70–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sousa TN, Kano FS, Brito CF, Carvalho LH. The Duffy binding protein as a key target for a Plasmodium vivax vaccine: lessons from the Brazilian Amazon. Mem Inst Oswaldo Cruz 2014; 0:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mangold KA, Manson RU, Koay ES, et al. . Real-time PCR for detection and identification of Plasmodium spp. J Clin Microbiol 2005; 43:2435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Turner L, Lavstsen T, Berger SS, et al. . Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 2013; 498:502–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khunrae P, Dahlbäck M, Nielsen MA, et al. . Full-length recombinant Plasmodium falciparum VAR2CSA binds specifically to CSPG and induces potent parasite adhesion-blocking antibodies. J Mol Biol 2010; 397:826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ntumngia FB, Schloegel J, Barnes SJ, et al. . Conserved and variant epitopes of Plasmodium vivax Duffy binding protein as targets of inhibitory monoclonal antibodies. Infect Immun 2012; 80:1203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cham GK, Kurtis J, Lusingu J, Theander TG, Jensen AT, Turner L. A semi-automated multiplex high-throughput assay for measuring IgG antibodies against Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) domains in small volumes of plasma. Malar J 2008; 7:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nielsen MA, Resende M, de Jongh WA, et al. . The influence of sub-unit composition and expression system on the functional antibody response in the development of a VAR2CSA based Plasmodium falciparum placental malaria vaccine. PLoS One 2015; 10:e0135406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trager W, Jensen JB. Human malaria parasites in continuous culture. Science 1976; 193:673–5. [DOI] [PubMed] [Google Scholar]
- 32. Srivastava A, Gangnard S, Round A, et al. . Full-length extracellular region of the var2CSA variant of PfEMP1 is required for specific, high-affinity binding to CSA. Proc Natl Acad Sci USA 2010; 107:4884–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wipasa J, Suphavilai C, Okell LC, et al. . Long-lived antibody and B Cell memory responses to the human malaria parasites, Plasmodium falciparum and Plasmodium vivax. PLoS Pathog 2010; 6:e1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang Q, Zhao Z, Zhang X, et al. . Naturally acquired antibody responses to Plasmodium vivax and Plasmodium falciparum merozoite surface protein 1 (MSP1) C-Terminal 19 kDa domains in an area of unstable malaria transmission in Southeast Asia. PLoS One 2016; 11:e0151900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Requena P, Arévalo-Herrera M, Menegon M, et al. . Naturally acquired binding-inhibitory antibodies to Plasmodium vivax Duffy binding protein in pregnant women are associated with higher birth weight in a multicenter study. Front Immunol 2017; 8:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Diggs CL, Sadun EH. Serological cross reactivity between Plasmodium vivax and Plasmodium falciparum as determined by a modified fluorescent antibody test. Exp Parasitol 1965; 16:217–23. [DOI] [PubMed] [Google Scholar]
- 37. Nagao Y, Kimura-Sato M, Chavalitshewinkoon-Petmitr P, et al. . Suppression of Plasmodium falciparum by serum collected from a case of Plasmodium vivax infection. Malar J 2008; 7:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kumar N, Folgar JP, Lubega P. Recognition of Plasmodium falciparum asexual stage antigens by antibodies in sera from people exposed to Plasmodium vivax.Am J Trop Med Hyg 1992; 47:422–8. [DOI] [PubMed] [Google Scholar]
- 39. Chuangchaiya S, Jangpatarapongsa K, Chootong P, et al. . Immune response to Plasmodium vivax has a potential to reduce malaria severity. Clin Exp Immunol 2010; 160:233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Costa JD, Zanchi FB, Rodrigues FL, et al. . Cross-reactive anti-PfCLAG9 antibodies in the sera of asymptomatic parasite carriers of Plasmodium vivax. Mem Inst Oswaldo Cruz 2013; 108:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Woodberry T, Minigo G, Piera KA, et al. . Antibodies to Plasmodium falciparum and Plasmodium vivax merozoite surface protein 5 in Indonesia: species-specific and cross-reactive responses. J Infect Dis 2008; 198:134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Igonet S, Vulliez-Le Normand B, Faure G, et al. . Cross-reactivity studies of an anti-Plasmodium vivax apical membrane antigen 1 monoclonal antibody: binding and structural characterisation. J Mol Biol 2007; 366:1523–37. [DOI] [PubMed] [Google Scholar]
- 43. Cao Y, Bansal GP, Merino K, Kumar N. Immunological cross-reactivity between malaria vaccine target antigen P48/45 in Plasmodium vivax and P. falciparum and cross-boosting of immune responses. PLoS One 2016; 11:e0158212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ntumngia FB, Thomson-Luque R, Torres Lde M, Gunalan K, Carvalho LH, Adams JH. A novel erythrocyte binding protein of Plasmodium vivax suggests an alternate invasion pathway into Duffy-positive reticulocytes. mBio 2016; 7:e01261–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.