Liver damage, endothelial activation, and hemostatic dysfunction are associated with a fatal outcome following Rift Valley fever virus infection, whereas having an elevated level of the chemokine RANTES (CCL-5) is associated with survival.
Keywords: Rift Valley, fever virus, biomarker, chemokine, inflammation, pathogenesis
Abstract
Rift Valley fever virus is an arbovirus found in Africa and the Middle East. Most infected individuals experience a mild self-limiting illness; however, some develop severe disease including hepatitis, hemorrhagic fever, or encephalitis. The biological reasons for these marked differences in disease manifestation are unknown. In this study, we evaluate 32 biomarkers in serum of 26 patients from an outbreak that occurred in Saudi Arabia in 2000–2001. Eleven biomarkers correlated with viral RNA. Thirteen biomarkers were associated with a fatal outcome. No associations of biomarkers and hemorrhage or central nervous system disease were identified in this cohort.
Rift Valley fever virus (RVFV) infection causes disease in both livestock and humans. In humans, disease is typically mild and self-limiting. However, in some individuals, manifestations of disease can be severe and can include hemorrhage or central nervous system (CNS) involvement. In fact, of the 886 cases during the RVFV outbreak that occurred in Saudi Arabia in 2000–2001, 7.1% had hemorrhagic manifestations, and 17.1% had neurologic manifestations; the case fatality rate was 13.9% [1]. Increased disease severity in some individuals may be secondary to differences in the quality of innate immune responses, as polymorphisms in Toll-like receptor (TLR) 8 and Retinoic acid inducible gene-I were noted in patients with neurologic manifestations, and polymorphisms in TLR3, TLR7, TLR8, and MyD88 were associated with hemorrhagic manifestations [2]. These proteins are all involved in sensing pathogen-associated molecular patterns and initiating or participating in downstream signaling events that trigger interferon and cytokine/chemokine responses. Previous work evaluating cytokines and chemokines during human RVFV infection is limited to only a handful of studies [3, 4], but these demonstrated associations between fatal outcomes and elevated levels of interleukin (IL)-8, CXCL9, Monocyte chemoattractant protein-1 (MCP)-1, interferon gamma inducible protein-10, IL-1α, IL-1 receptor antagonist, and IL-10. This suggests that innate signaling mechanisms could contribute to RVFV pathogenesis. We recently conducted a longitudinal study on 3 patients with RVFV disease with hemorrhagic manifestations and identified several novel biomarkers that were elevated compared to healthy controls [5]. In this study, we sought to extend upon those observations and determine any associations between disease manifestations and/or outcome with any of these newly identified biomarkers.
METHODS
This study used de-identified, preexisting specimens and clinical data obtained during an outbreak that occurred in Saudi Arabia in 2000–2001. All patients were identified as having acute Rift Valley fever by either quantitative reverse-transcription polymerase chain reaction (qRT-PCR), viral antigen capture test, or a positive RVFV immunoglobulin M result as previously reported [6]. The Centers for Disease Control and Prevention (CDC) Human Research Protection Office granted an exemption from full review (CDC protocol number 6920).
Serum samples that had been collected for RVFV diagnostic purposes were analyzed using commercial multiplex assays according to the manufacturer’s instructions. The samples were γ-irradiated with 5 × 106 rads prior to analysis. Twenty-eight analytes were assessed in 8 commercially available assays from Invitrogen (Carlsbad, California), Millipore (Billerica, Maryland), and Affymetrix (Santa Clara, California). The largest of these was a 13-plex assay for interferon-beta (IFN–β), tissue plasminogen activator, IL-29, regulated on activation normal T-cell–expressed and secreted (RANTES, CCL-5), hepatocyte growth factor (HGF), interferon alpha (IFN-α), monokine induced by γ-interferon (CXCL-9), P-selectin, sFas-ligand, E-selectin, plasminogen activator inhibitor-1, tumor necrosis factor receptor I (TNFRI), and granzyme B. Additional multiplex assays included a 4-plex assay for D-dimer, L-selectin, intercellular adhesion molecule (ICAM), and vascular cell adhesion molecule; a 2-plex assay for (MCP-2, CCL-8) and TNFRII; a 2-plex assay for tissue factor and thrombomodulin; and a 4-plex assay for von Willebrand factor, C-reactive protein, fibrinogen, and platelet factor (PF4) (Millipore). Single-plex assays were performed for ferritin, a disintegrin and metalloproteinase with thrombospondin motifs 13 (ADAMTS-13), and complement factor H. Data were collected on a Luminex 200 (Austin, Texas), and all assay results were reported as picograms per milliliter or nanograms per milliliter. PF4 levels were near or below the limit of detection in all patient samples, so data are not presented graphically. Fibrinogen data were excluded since serum samples by definition are devoid of fibrinogen. The tissue factor assay measured levels, not activity, and many samples were at or below the limit of detection. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), and platelet counts were obtained from the database collected during the outbreak. Patient biomarkers were analyzed for correlation with RVFV viral RNA load (raw cycle threshold [Ct] value) using Spearman rank correlation. Given the nonnormal distribution of the data, biomarker data were analyzed using the Wilcoxon rank-sum test, comparing the differences in each of the 32 biomarkers for the following clinical categories: presence of hemorrhage (with or without), CNS involvement (with or without), and outcome (fatal or nonfatal). The false discovery rate as developed by Benjamini and Hochberg was used to determine the statistical significance of each biomarker with an overall α = .05 to control for multiple comparisons. Figures were generated using Prism. Biomarkers that were statistically significant are shown in the main manuscript; the remainder of the data are presented in the Supplementary Figure.
RESULTS
The patient samples used in this study were collected during an RVFV outbreak that occurred in Saudi Arabia in 2000–2001. The CDC assisted with outbreak response and provided diagnostic support. The residual serum samples were stored in liquid nitrogen. Previous work using similar specimens from the same outbreak found that those who died of the infection exhibited higher viral loads [6]. Patients’ ages ranged from 17 to 90 years; the cohort consisted of 20 men and 6 women. All patients were hospitalized, so this group already represents individuals who were more severely affected by RVFV infection. Most patients experienced fever and gastrointestinal symptoms, 8 had CNS manifestations (confusion, lethargy, and/or disorientation), and 3 had hemorrhagic manifestations (hematemesis or rectal bleeding); 6 succumbed to infection (Supplementary Table 1).
Only 1 sample was available from a single time point for each patient, so to evaluate for bias related to time of sampling, we compared the average day post–symptom onset on which each sample was obtained with the presence or absence of hemorrhagic manifestations (average days after onset with hemorrhage, 5 [range, 3–7]; without hemorrhage, 4.8 [range, 1–14]), CNS involvement (average days after onset with CNS disease, 5.1 [range, 3–8]; without CNS disease, 4.7 [range, 1–14]), and outcome (average days after onset fatal outcome, 4.5 [range, 3–8]; nonfatal outcome, 5 [range, 1–14]).
Raw Ct values (quantitative measure of viral RNA load by qRT-PCR) were available from the prior analysis of this cohort [6], so Ct value was compared to the measured biomarkers to evaluate for correlation. D-dimer, ALT, ICAM, L-selectin, HGF, E-selectin, AST, TNFRII, TNFRI, and tissue factor all had a negative correlation with Ct, hence a positive correlation with viral RNA load. Only RANTES demonstrated a positive correlation with Ct value, hence a negative correlation with viral RNA load (Table 1).
Table 1.
Correlation With Rift Valley Fever Virus Cycle Threshold Value
| Field Name | Spearman Correlation Coefficient | P Value |
|---|---|---|
| D-dimer | –0.7653 | <.001 |
| ALT | –0.5767 | .003 |
| ICAM | –0.5425 | .003 |
| L-selectin | –0.5382 | .004 |
| HGF | –0.5051 | .006 |
| E-selectin | –0.5047 | .006 |
| AST | –0.5140 | .007 |
| TNFRII | –0.4892 | .007 |
| TNFRI | –0.4864 | .008 |
| RANTES (CCL-5) | 0.4804 | .008 |
| Tissue factor | –0.4466 | .014 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; HGF, hepatocyte growth factor; ICAM, intercellular adhesion molecule; RANTES, regulated on activation normal T-cell expressed and secreted; TNFR, tumor necrosis factor receptor.
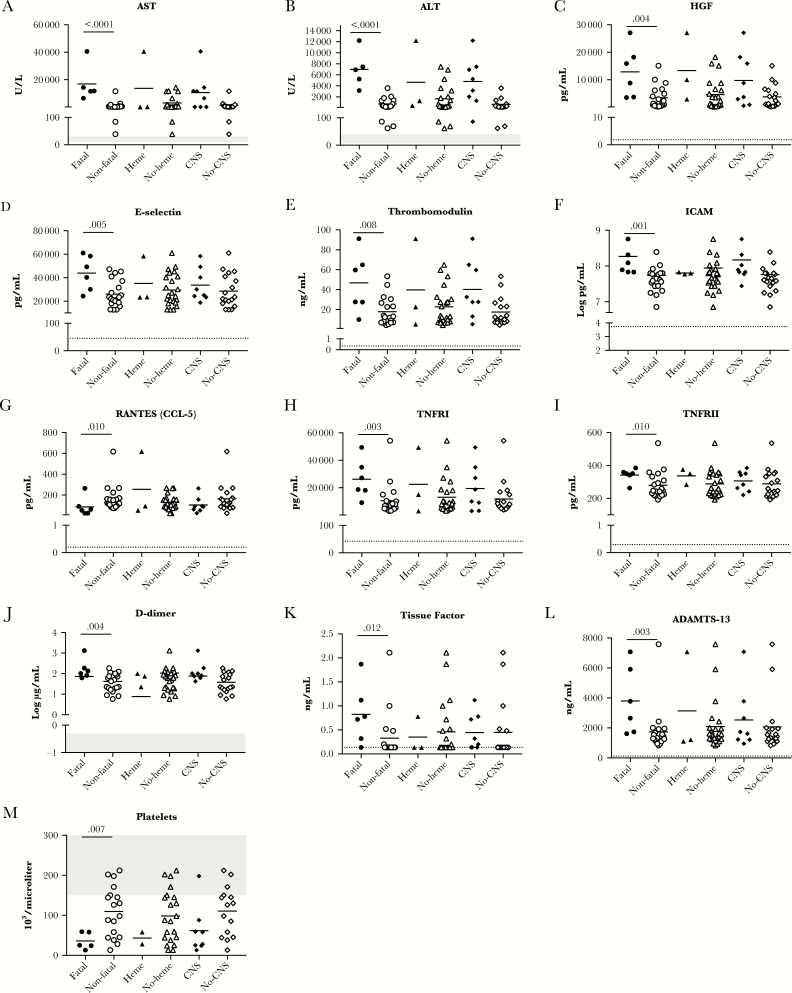
As has been previously reported for RVFV infection, individuals with a fatal outcome had elevated levels of the hepatic aminotransferases AST and ALT (Figure 1A and 1B). Consistent with evidence of liver damage during RVFV infection is the finding of elevated levels of HGF in patients with a fatal outcome as compared to those who survived (Figure 1C). Notably, for ALT, AST, and HGF the data are markedly skewed by one outlier; this was the one patient in the cohort with both CNS disease and hemorrhagic manifestations who also died.
Figure 1.
A–M, Biomarkers with a significant association with fatal outcome. Biomarker levels are depicted as a function of outcome, hemorrhagic manifestations, or central nervous system manifestations. All individual data points are shown; the bar is the mean. Numbers above the data sets represent the P value as an indication of the statistical significance of the difference. Normal values are indicated on the graphs with gray shading in cases in which standard clinical ranges are established. The dotted line depicts the limit of detection of the assay. For tissue factor, many samples did not have any detectable tissue factor; these data are plotted at the limit of detection. Abbreviations: ADAMTS-13, a disintegrin and metalloproteinase with thrombospondin motifs 13; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CNS, central nervous system; HGF, hepatocyte growth factor; ICAM, intercellular adhesion molecule; RANTES, regulated on activation normal T-cell expressed and secreted; TNFR, tumor necrosis factor receptor.
Three markers of endothelial function, E-selectin, thrombomodulin, and ICAM, were higher in patients with a fatal outcome (Figure 1D–F). RANTES demonstrated an inverse correlation with viral RNA load (Table 1), and there were significantly higher levels of RANTES in patients who survived (Figure 1G). TNFRI and TNFRII were significantly higher in patients who had a fatal outcome (Figure 1H and 1I). Finally, D-dimer, tissue factor, and ADAMTS-13, all markers of hemostatic pathways, were higher in patients with a fatal outcome, whereas thrombocytopenia was more marked in patients with a fatal outcome (Figure 1J–M).
DISCUSSION
In this study we confirm and expand on previously known features of severe RVFV disease. Consistent with the known liver tropism of RVFV leading to hepatic damage, we observed an association between elevated aminotransferases and fatal outcome, as previously reported [1]. Additionally, there was a correlation between viral RNA load and several markers of hepatic function: AST, ALT, and HGF. HGF is produced after liver injury and is a potent cytokine that stimulates regeneration [7]. In critically ill patients with liver disease, hemorrhage is a common sequela secondary to loss of hepatic synthetic function. Therefore, separating liver disease from hemorrhagic manifestations is difficult, as the liver is responsible for producing several important coagulation factors. We were unable to conduct an extensive assessment of coagulation factors in this study, so the etiology of the hemorrhage seen in RVFV patients is unknown. However, it is notable that D-dimer and tissue factor levels correlated with viral RNA load and demonstrated an association with a fatal outcome, suggesting that infection modulates factors involved in hemostatic pathways. Additionally, we found that thrombocytopenia was associated with a fatal outcome, consistent with previous reports [1]. We also previously reported an association between survival from RVFV disease and elevated levels of sCD40L, the majority of which is derived from activated platelets [3]. These data are complementary and underscore the complex interplay between immunity and coagulation pathways.
We found evidence of endothelial activation and dysfunction in patients with RVFV disease. Selectins and adhesion molecules are upregulated on activated endothelial surfaces and facilitate interactions with leukocytes in the blood to attract them to sites of inflammation [8]. There was a correlation between RVFV RNA load and ICAM, L-selectin, and E-selectin, implicating virally driven endothelial dysfunction in RVFV disease. Thrombomodulin present in the microenvironment on the endothelial surface inhibits coagulation by activating protein C, but is shed from the endothelium upon activation [9]. Three markers of endothelial activation, thrombomodulin, E-selectin, and ICAM, were all associated with a fatal outcome, emphasizing the role of endothelial activation in pathogenesis of RVFV. Endothelial activation is also a feature of other viral hemorrhagic fevers such as Ebola and dengue [9, 10].
The only biomarker that showed an inverse correlation with viral RNA load was the chemokine RANTES. This is quite interesting, because in one other study of patients with RVFV disease, lower than normal levels of RANTES were seen in patients with a fatal outcome [4], and we also detected an association between low RANTES levels and a fatal outcome. RANTES is a chemokine that is important for normal T-cell function [11], so this implies that T-cell function could be an important part of RVFV control. However, the field is lacking in data on human T-cell responses to RVFV; this is an area that is worthy of additional research focus.
The role of soluble TNFRI/II in RVFV is unknown. It has been hypothesized that it impairs the host inflammatory response by neutralizing free TNF-α [12]. Elevated levels of soluble TNFRI/II levels have been reported in other inflammatory diseases [13], so our finding of elevated soluble TNFRI/II in patients with fatal RVFV disease indicates a deleterious perturbation in this pathway in association with disease outcome.
This study is limited by small sample size, and lack of longitudinal analysis, since only one sample was available from each patient. However, these data suggest that several mechanisms of support could be considered in the care of RVFV patients. First, the pivotal role of liver infection and inflammation in severe RVFV disease suggests that patients could possibly benefit from interventions limiting hepatic damage. Early administration of antiviral medications could be helpful, but concerns exist for skewing the balance toward encephalitic disease with antiviral administration, as observed with ribavirin treatment [14]. An alternative that has shown some promise in dengue virus–related hepatic disease is N-acetylcysteine (NAC) [15]. NAC has a favorable safety profile and is commonly used to protect the liver from drug-related toxicity after acetaminophen overdose, but some also report success in using NAC to treat virally mediated hepatitis. Second, early platelet transfusion could improve outcomes, especially in patients with thrombocytopenia. Finally, interventions aimed at stabilizing the endothelium and decreasing its activation, such as anti-inflammatory agents, could also be beneficial.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Institutes of Health (grant number K08 AI119448); the Burroughs Wellcome Fund (Career Award for Medical Scientists number 1013362.01); and the Children’s Healthcare of Atlanta Pediatric Research Trust (to A. K. M.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1. Madani TA, Al-Mazrou YY, Al-Jeffri MH, et al. . Rift Valley fever epidemic in Saudi Arabia: epidemiological, clinical, and laboratory characteristics. Clin Infect Dis 2003; 37:1084–92. [DOI] [PubMed] [Google Scholar]
- 2. Hise AG, Traylor Z, Hall NB, et al. . Association of symptoms and severity of rift valley fever with genetic polymorphisms in human innate immune pathways. PLoS Negl Trop Dis 2015; 9:e0003584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McElroy AK, Nichol ST. Rift Valley fever virus inhibits a pro-inflammatory response in experimentally infected human monocyte derived macrophages and a pro-inflammatory cytokine response may be associated with patient survival during natural infection. Virology 2012; 422:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jansen van Vuren P, Shalekoff S, Grobbelaar AA, et al. . Serum levels of inflammatory cytokines in Rift Valley fever patients are indicative of severe disease. Virol J 2015; 12:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de St Maurice A, Harmon J, Nyakarahuka L, et al. . Rift valley fever viral load correlates with the human inflammatory response and coagulation pathway abnormalities in humans with hemorrhagic manifestations. PLoS Negl Trop Dis 2018; 12:e0006460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bird BH, Bawiec DA, Ksiazek TG, Shoemaker TR, Nichol ST. Highly sensitive and broadly reactive quantitative reverse transcription-PCR assay for high-throughput detection of Rift Valley fever virus. J Clin Microbiol 2007; 45:3506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fajardo-Puerta AB, Mato Prado M, Frampton AE, Jiao LR. Gene of the month: HGF. J Clin Pathol 2016; 69:575–9. [DOI] [PubMed] [Google Scholar]
- 8. McEver RP. Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc Res 2015; 107:331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Page AV, Liles WC. Biomarkers of endothelial activation/dysfunction in infectious diseases. Virulence 2013; 4:507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McElroy AK, Erickson BR, Flietstra TD, et al. . Ebola hemorrhagic fever: novel biomarker correlates of clinical outcome. J Infect Dis 2014; 210:558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Makino Y, Cook DN, Smithies O, et al. . Impaired T cell function in RANTES-deficient mice. Clin Immunol 2002; 102:302–9. [DOI] [PubMed] [Google Scholar]
- 12. Giai C, Gonzalez C, Ledo C, et al. . Shedding of tumor necrosis factor receptor 1 induced by protein A decreases tumor necrosis factor alpha availability and inflammation during systemic Staphylococcus aureus infection. Infect Immun 2013; 81:4200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Munroe ME, Vista ES, Guthridge JM, Thompson LF, Merrill JT, James JA. Proinflammatory adaptive cytokine and shed tumor necrosis factor receptor levels are elevated preceding systemic lupus erythematosus disease flare. Arthritis Rheumatol 2014; 66:1888–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scharton D, Bailey KW, Vest Z, et al. . Favipiravir (T-705) protects against peracute Rift Valley fever virus infection and reduces delayed-onset neurologic disease observed with ribavirin treatment. Antiviral Res 2014; 104:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Senanayake MP, Jayamanne MD, Kankananarachchi I. N-acetylcysteine in children with acute liver failure complicating dengue viral infection. Ceylon Med J 2013; 58:80–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.