Abstract
Pleural effusions are a common medical problem not only for pulmonologists but also for general physicians, often needing thoracentesis for a definite diagnosis. However, thoracentesis cannot always reveal malignant cells or microbiological evidence.
In this context, we prospectively enrolled a total of 289 patients with pleural effusions due to diverse etiologies: parapneumonic effusion (PPE) (63), empyema (22), tuberculous pleural effusion (TBPE) (54), malignant pleural effusion (MPE) (140), or chronic renal failure (CRF)/congestive heart failure (CHF) (10). The MPE group consisted of lung cancer (adenocarcinoma, n = 90; squamous cell carcinoma, n = 5; small cell carcinoma, n = 4), malignant lymphoma (n = 17), malignant mesothelioma (n = 11), malignant melanoma (n = 3), and metastasis from other organs (n = 10).
This study demonstrated that the pleural lactate dehydrogenase (LDH)to adenosine deaminase (ADA) ratios differed significantly between patients with CHF/CRF, MPE, TBPE, empyema, and PPE. We discovered a simple method to differentiate pleural diseases based on the pleural LDH to ADA ratio and carcinoembryonic antigen (CEA). A pleural LDH to ADA ratio greater than 15.5 and a pleural CEA level of less than 5 ng/mL is indicative of PPE or empyema rather than TBPE, MPE, or transudative pleural effusion (CRF, CHF).
This method has a sensitivity of 62.0%, a specificity of 91.0%, and an area under the receiver operating characteristic curve of 0.765 (95% confidence interval [CI]: 0678–0.852, P < .001), odds ratio of 16.6 (95% CI: 7.28–37.8, P < .001), a positive likelihood ratio (LR) of 6.8, and a negative LR of 0.02.
Keywords: empyema, parapneumonic effusion, pleural effusion, pleural LDH/ADA ratio
1. Introduction
General physicians often encounter patients with pleural effusions, which include parapneumonic effusions (PPE), empyemas, tuberculous pleural effusions (TBPE), malignant pleural effusions (MPE), and those caused by chronic heart failure (CHF), and chronic renal failure (CRF). After the establishment of Light's criteria for discriminating exudative from transudative pleural effusions,[1] no simple methods for differentiating etiologies have been reported. The aim of this study is to evaluate the role of the pleural lactase dehydrogenase (LDH) to adenosine deaminase (ADA) ratio and the pleural carcinoembryonic antigen (CEA) levels in the differential diagnosis of the various etiologies.
2. Methods
We prospectively enrolled adult patients with pleural effusion between November 2012 and November 2016 in an outpatient or inpatient setting. Patients included in the study either displayed signs of pleural fluid at the time of their first visit to the Kyorin University Hospital (a 1100-bed tertiary care center in Tokyo), or developed pleural effusion during hospitalization. Informed consent was obtained from all patients. This study was approved by the Ethics Board of Kyorin University (approval number H24-080-04).
The definition of empyema was based on Light's criteria (including only classes 6 and 7)[2] and patients with PPE were in classes 1 to 5. TBPE was defined by an ADA level >40 U/L, together with regression or resolution of the pleural effusion after initiation of antituberculosis therapy, the presence of epithelioid granuloma in pleural biopsies, or the presence of Mycobacterium tuberculosis in the pleural fluid confirmed by microbiological tests, including polymerase chain reaction. MPE was diagnosed when the pleural fluid was cytologically positive for malignant cells. CHF and/or CRF were diagnosed through clinical examination.
To evaluate semiquantitatively the total amount of pleural effusion, chest X-rays were inspected at 3 levels:
-
(1)
bronchial bifurcation,
-
(2)
upper level of the diaphragm, and
-
(3)
midway between those levels as described in previous report.[3]
The degree of fluid accumulation was defined as mild, moderate, or severe depending on whether the fluid observed in the chest X-ray rose higher than level 1, 2, or 3, respectively. Patients who showed vertically oriented pleural effusion with pleural adhesion or had no chest X-ray were excluded, because of the difficulty in measuring the amount of fluid in such cases.
Statistical comparisons were performed using the non-parametric Mann–Whitney test. All tests were 2-sided. A P-value less than .05 was considered statistically significant. The cut-off point for the markers in the pleural fluid was determined as the minimum value of [(1-sensitivity)2 + (1-specificity)2]. Data were analyzed using SPSS software (IBM com, Japan), version 25.0 for Windows.
3. Results
A total of 289 patients had pleural effusions with the following etiologies: PPE (63), empyema (22), TBPE (54), MPE (140), or CRF/CHF (10). The MPE group consisted of lung cancer (adenocarcinoma, n = 90; squamous cell carcinoma, n = 5; small cell carcinoma, n = 4), malignant lymphoma (n = 17), malignant mesothelioma (n = 11), malignant melanoma (n = 3), and metastasis from other organs (n = 10). Among TBPE patients, microbiological or pathological confirmation was obtained in 18.2% (n = 10) and 7.3% (n = 4) of the cases, respectively. Using Light's criteria, the proportion of exudative pleural effusion in CRF/CHF, MPE, TBPE, PPE, and empyema was 20%, 96.4%, 100%, 100%, and 100%, respectively.
3.1. Semiquantitative analysis of the total amount of pleural fluid from chest X-rays
The total amount of pleural effusion was evaluated for each patient based on the chest X-ray classification (Fig. 1) as described in the Methods. The proportion of mild and moderate effusions was comparable between the groups. However, the proportion of patients with severe pleural effusion was significantly higher in MPE (n = 34, 24.5%, P = .036), empyema (n = 7, 31.8%, P = .027), and PPE (n = 15, 25.4%, P = .042) than in TBPE (n = 4, 9.1%).
Figure 1.
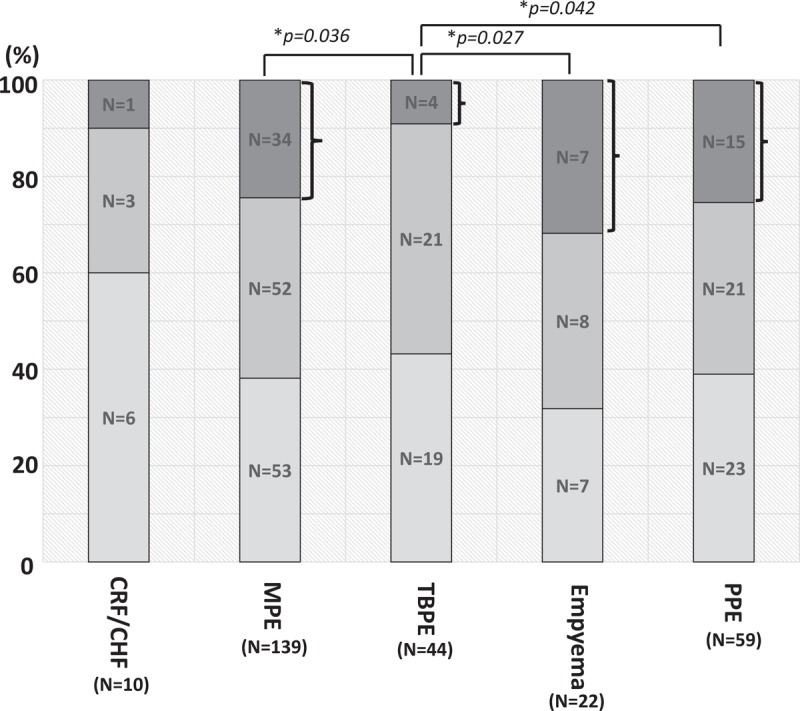
Proportion of patients with mild, moderate and severe pleural effusion on chest X-ray. The proportion of patients with severe pleural effusion was significantly higher in MPE (n = 34, 24.5%, P = .036), empyema (n = 7, 31.8%, P = .027), and PPE (n = 15, 25.4%, P = .042) than in TBPE (n = 4, 9.1%). ∗Means P < .05. MPE = malignant pleural effusion, PPE = parapneumonic effusion, TBPE = tuberculous pleural effusion.
3.2. Analysis of LDH to ADA ratio in pleural fluid
Interestingly, the correlation between pleural LDH and pleural ADA was statistically significant in PPE (r = 0.688, P < .001), empyema (r = 0.964, P < .001), TBPE (r = 0.425, P = .001), MPE (r = 0.635, P < .001,), and CHF/CRF (r = 0.537, P = .016; Fig. 1). Furthermore, pleural LDH to ADA ratios differed significantly between patients with CHF/CRF (median: 13.0, interquartile range [IQR]: 8.3–16.2), MPE (median: 21.6, IQR: 14.0–32.5), TBPE (median: 5.1, IQR: 3.7–7.9), empyema (median 50.0, IQR: 37.2–77.9), and PPE (median 35.4, IQR: 19.6–47.3; Fig. 2). Receiver operating characteristic (ROC) curve analysis showed that the pleural LDH to ADA ratio can differentiate the PPE/empyema groups from non-PPE/empyema groups (CHF/CRF, TBPE, MPE), with an area under the curve (AUC) of 0.783 (95% confidence interval [CI]: 0.724–0.842, P = .03). In particular, if the threshold of the pleural LDH to ADA ratio was set at 15.5, PPE/empyema could be discriminated from other causes with a sensitivity of 87.1%, a specificity of 49.5%, a likelihood ratio (LR) of 1.72, an AUC of 0.683 (95% CI: 0.619–0.746, P < .001), and an odds ratio of 6.6 (95% CI: 3.3–13.2, P < .001) (Table 1).
Figure 2.
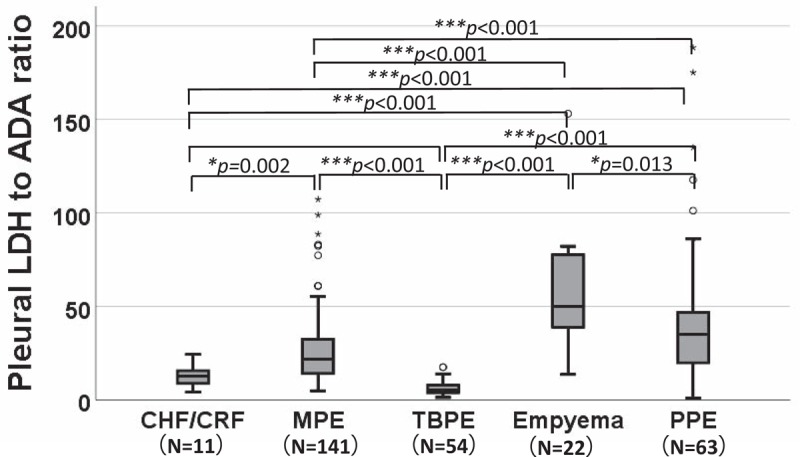
Comparison of the pleural LDH to ADA ratio in various diseases. ∗Means P < .05, ∗∗Means P < .01, ∗∗∗Means P < .001. ADA = adenosine deaminase, CHF/CRF = chronic heart failure/chronic renal failure, LDH = lactase dehydrogenase, MPE = malignant pleural effusion, PPE = parapneumonic effusion, TBPE = tuberculous pleural effusion.
Table 1.
Diagnostic accuracy of combination of various parameters for empyema and PPE.
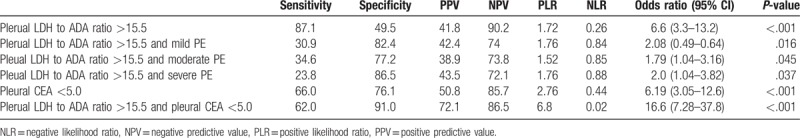
3.3. Diagnostic accuracy for empyema/PPE by using various parameters; pleural LDH to ADA ratio >15.5, pleural CEA, and semiquantitative analysis of pleural fluid on chest X-ray
ROC curve analysis demonstrated that pleural CEA could discriminate MPE from other diseases with an AUC of 0.806 (95% CI: 0.745–0.867, P < .001) (Fig. 3). The optimal threshold for pleural CEA was 5.3 ng/mL, corresponding to sensitivity, specificity, and positive LR of 81.1%, 75%, and 3.24, respectively (Fig. 3, dotted circle).
Figure 3.

Pleural CEA can discriminate MPE from other diseases with an area under the receiver operating characteristic curve (AUC) of 0.806 (95% CI: 0.745–0.867, P < .001). The dotted circle represents a cut-off of 5.3 ng/mL in pleural CEA. AUC = area under the curve, CEA = carcinoembryonic antigen, CI = confidence interval.
We evaluated the diagnostic accuracy for empyema/PPE by using various parameters: Pleural LDH to ADA ratio >15.5, pleural CEA <5 ng/mL, and the degree of pleural fluid accumulation on chest X-ray (Table 1). Using the first 2 parameters, we could differentiate empyema/PPE from other diseases with a sensitivity of 62%, specificity of 91%, positive LR of 6.8, negative LR of 0.02, and odds ratio of 16.6 (95% CI; 7.28–37.8, P < .001). On the contrary, combining the pleural LDH to ADA ratio with the degree of pleural effusion on chest X-rays showed much weaker statistical power to discriminate empyema/PPE from other diseases (Table 1).
4. Discussion
Although no simple method exists to discriminate empyema/PPE from other diseases, we have previously reported the usefulness of the pleural LDH to ADA ratio[3,4] for this differential diagnosis. In this regard, the present study demonstrates that the pleural LDH to ADA ratio differs in various diseases, including the infectious ones, as it was significantly lower in TBPE than in MPE, PPE and empyema (Fig. 2). Furthermore, we found that the combination of 2 criteria (pleural LDH to ADA ratio >15.5 and CEA <5 ng/mL) allows a more accurate diagnosis of empyema/PPE.
Massive pleural effusions mostly occur in MPE, PPE/empyema, and TBPE[3]: using semiquantitative analysis we found that the proportion of severe pleural fluid was significantly higher in MPE, PPE, and empyema compared to TBPE. The pathogenesis of increased pleural fluid elevation can be attributed to the possible increase in cytokines associated with inflammation (ie, interleukin-8) and/or permeability (ie, vascular endothelial growth factor, transforming growth factor-β) in the pleural cavity.[3] However, the 2 parameters (pleural LDH to ADA ratio >15.5 and the degree of pleural effusion) cannot be used as reliable markers to discriminate empyema/PPE from other diseases.
This study has some limitations:
-
(1)
a relatively small number of patients were included, and
-
(2)
only 25.9% (n = 14) of TBPE cases were confirmed by pathological or microbiological tests.
However, physicians usually handle pleural effusion cases without any definite evidence from cytological, pathological, or microbiological evaluations. Therefore, this novel method using the combination of pleural LDH to ADA ratio and CEA levels can be an effective diagnostic tool in addition to Light's criteria.[5] Our result needs to be confirmed in future studies with the analysis of more cases, but this simple method can have practical applicability in the etiological assessment of pleural effusions.
Author contributions
Conceptualization: Hajime Takizawa, Richard W. Light.
Data curation: Takeshi Saraya, Kosuke Ohkuma, Takashi Koide, Hajime Goto.
Formal analysis: Takeshi Saraya.
Investigation: Takeshi Saraya, Kosuke Ohkuma, Takashi Koide, Hajime Takizawa.
Project administration: Kosuke Ohkuma, Takashi Koide, Hajime Takizawa.
Supervision: Richard W. Light.
Writing – original draft: Takeshi Saraya, Hajime Goto.
Writing – review and editing: Richard W. Light.
Takeshi Saraya orcid: 0000-0003-0502-8128.
Footnotes
Abbreviations: ADA = adenosine deaminase, CEA = carcinoembryonic antigen, CHF = chronic heart failure, CRF = chronic renal failure, LDH = lactate dehydrogenase, MPE = malignant pleural effusion, PPE = parapneumonic effusion, TBPE = tuberculous pleural effusion.
The authors have no conflicts of interest to disclose.
References
- [1].Light RW, Macgregor MI, Luchsinger PC, et al. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972;77:507–13. [DOI] [PubMed] [Google Scholar]
- [2].Light RW. Clinical practice. Pleural effusion. N Engl J Med 2002;346:1971–7. [DOI] [PubMed] [Google Scholar]
- [3].Saraya T, Ohkuma K, Watanabe T, et al. Diagnostic value of vascular endothelial growth factor, transforming growth factor-beta, interleukin-8, and the ratio of lactate dehydrogenase to adenosine deaminase in pleural effusion. Lung 2018;196:249–54. [DOI] [PubMed] [Google Scholar]
- [4].Tsujimoto N, Saraya T, Light RW, et al. A simple method for differentiating complicated parapneumonic effusion/empyema from parapneumonic effusion using the split pleura sign and the amount of pleural effusion on thoracic CT. PLoS One 2015;10:e0130141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Feller-Kopman D, Light R. Pleural disease. N Engl J Med 2018;378:740–51. [DOI] [PubMed] [Google Scholar]


