Abstract
Background:
Impaired language function is frequently observed as an initial sign in people with autism spectrum disorder (ASD). However, clinically, the early stages of ASD are difficult to distinguish from those of developmental language disorder (DLD).
Objective:
To evaluate the ability of diffusion tensor imaging (DTI) parameters for language-related white matter tracts (arcuate fasciculus) to differentiate ASD from DLD among toddlers.
Materials and methods:
We included 16 ASD toddlers with language delay and 18 DLD toddlers in this study. Magnetic resonance imaging sequences included T2-weighted imaging (T2WI), T1 3-dimensional magnetization-prepared rapid acquisition gradient-echo (3D MP-RAGE), and DTI. Tractography was performed using Neuro 3D in the Siemens Syngo Workstation, and fractional anisotropy (FA), average fiber length (AFL), tract volume (TV), and number of voxels (NV) were automatically calculated. Data were then analyzed using IBM SPSS Statistics 22.
Results:
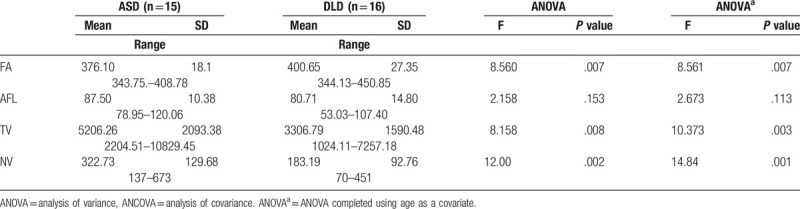
The ASD group exhibited significantly lower FA values, as well as significantly higher TV and NV values compared with the DLD group. With age as the covariate, analysis of covariance revealed different significances in TV and NV. Analysis of variance for AFL revealed no significant differences between the 2 groups.
Conclusion:
DTI parameters of arcuate fasciculus were useful for differentiating ASD with language delay from DLD among toddlers. DTI has the potential to provide an objective and effective method for aiding early diagnosis, early intervention and improving long-term outcomes of ASD.
Keywords: autism spectrum disorder, developmental language disorder, diffusion tensor imaging, language-related white matter tract, toddler
1. Introduction
Autism, Asperger's syndrome, Rett syndrome and disorder—not otherwise specified (PDD-NOS) are a group of neurodevelopmental pathologies known collectively as autism spectrum disorder (ASD). ASD is characterized by severe impairment in reciprocal social interactions and communication skills, and the presence of restricted, stereotypical behavior.[1] Impaired language function is frequently observed as an initial sign in people with ASD. The incidence of ASD has steadily increased in recent years, and international epidemiological surveys have reported an incidence of ASD ranging from 60/10,000 to 100/10,000 people.[2,3]
The mechanisms underlying ASD remain largely unknown. In recent years, methods for evaluating the behavioral characteristics of children with ASD have been based on several rating-scale instruments and qualitative analysis of the complexity of affecting factors.[4,5] The commonly used scales include Diagnostic and Statistical Manual of Mental Disorders Fourth Edition Text Revision (DSM), Childhood Autism Rating Scale (CARS), and Autism Behavior Checklist (ABC). The initial presentation of ASD with language delay is similar to that of developmental language disorder (DLD), and the 2 disorders are difficult to be differentiated clinically. Because ASD and DLD require substantially different treatment, the development of objective and sensitive methods for diagnosing ASD in the early stages in clinical practice is an important research area.
As a well-developed theory of the mechanisms underlying ASD, the weak central coherence theory was devised by Frith in 1994,[6] and has become widely accepted in recent decades.[7–9] The symptoms of ASD have been hypothesized to be caused by changes in brain connectivity. This connectivity involves white matter tracts that link discrete grey matter regions into integrated neural circuits. The arcuate fasciculus (AF) is an important language-related white matter tract connecting the frontal expressive language area with the posterior temporoparietal receptive language areas, forming the substrate of the dorsal language pathway.
Diffusion tensor imaging (DTI) is a noninvasive, highly sensitive method for delineating WM tracts and providing indirect quantitative measures of WM integrity. Wakana and Smits reported a method for examining the AF.[10,11] Although a number of studies have examined language-related white matter tracts using DTI, relatively few DTI studies have been conducted with young children. Given the importance of early intervention in ASD, finding efficient methods for early diagnosis is an urgent research aim. As an objective method, DTI may provide an important tool for assisting in the clinical diagnosis of ASD. In the present study, we focused on AF and hypothesize that the imaging features of AF may be different between ASD toddlers with language delay and DLD toddlers. DTI was used to investigate AF alterations of ASD and DLD.
2. Materials and methods
2.1. Subjects
We examined 16 toddlers with ASD with language delay (11 males, 5 females; mean age 2.41 ± 0.58 years, range 1.42–3.25 years), and 18 toddlers with DLD (11 males, 7 females; mean age 2.46 ± 0.63 years, range 1.25–3.83 years). Diagnosis of toddlers with ASD was conducted by 2 experienced neuropsychologists using the Autism Behavior Checklist-ABC. This scale has 5 subscales: Irritability, Lethargy-Social Withdrawal, Stereotypic Behavior, Hyperactivity, and Speech Disorder. All ASD toddlers exhibited an ABC score of at least 30 (average ABC score 49.9 ± 8.0) and presented clinical symptoms of stereotypes, communication disorders, and/or language regression. The ABC scores of all DLD toddlers were less than 30, with an average ABC score of 22.9 ± 3.8. The 2 groups were well matched in terms of age, gender, and handedness. The assessment of language function was based on the Expressive Language and Receptive Language subsets of the Chinese Version of the Psycho-Educational Profile (C-PEP). The C-PEP scale not only evaluates children with ASD and DLA but also develops individualized training programs for clinicians and parents. It includes 95 functional development scales and 44 pathological scales. This study used this scale to assess the language function. Mean intelligence quotient (IQ) was measured using the Wechsler Intelligence Scale for Children (WISC), which include 14 subtests. Toddlers with hypoxic-ischemic brain damage (HIBD), brain trauma and other congenital or acquired defects were excluded. This study was approved by the local institutional review board, and informed consent was obtained from all parents before the study began.
2.2. Magnetic resonance imaging (MRI) protocol
Magnetic resonance imaging (MRI) scans were acquired with a 3.0 T Siemens TIM Trio scanner. MRI sequences included T2-weighted imaging (T2WI), 3-dimensional magnetization-prepared rapid acquisition gradient-echo (T1 3D MP-RAGE) and single-shot echo planar (SE-EPI) imaging. The imaging parameters were as follows: T2WI: repetition time (TR) = 3220 ms, echo time (TE) = 99 ms, field of view (FOV) = 250 mm, slices = 20, slice thickness = 5.0 mm. T1 3D MP-RAGE: TR = 1900 ms, TE = 2.5 ms, FOV = 250 mm, slices = 176, slice thickness = 1.0 mm, and bandwidth = 170. DTI: TR = 5500 ms, TE = 92 ms, FOV = 260 mm, matrix = 128 × 128, 20 diffusion encoding directions, slice thickness = 3.0 mm, and variable b-values between 0 and 1000 s/mm2. Toddlers were sedated using chloral hydrate (0.5 g in 10 mL) during MRI scanning.
3. Data analysis
For tractography, post-processing was performed using Neuro 3D in the Siemens Syngo Workstation. Tractography of the AF was performed by 2 raters. After fusing the images between T1 3D MP-RAGE and DTI, the AF was tracked by placing a seed region of interest (ROI) in the green triangular-shaped periventricular white matter on the encoded tensor map in the coronal plane and a target ROI in the posterior temporal lobe, shown as a blue narrow strip structure lateral to the splenium of the corpus callosum. Using this method, we performed tract reconstruction of the AF separately (Fig. 1A andFig. 1B). Fractional anisotropy (FA), average fiber length (AFL), tract volume (TV) and number of voxels (NV) were then automatically calculated.
Figure 1.
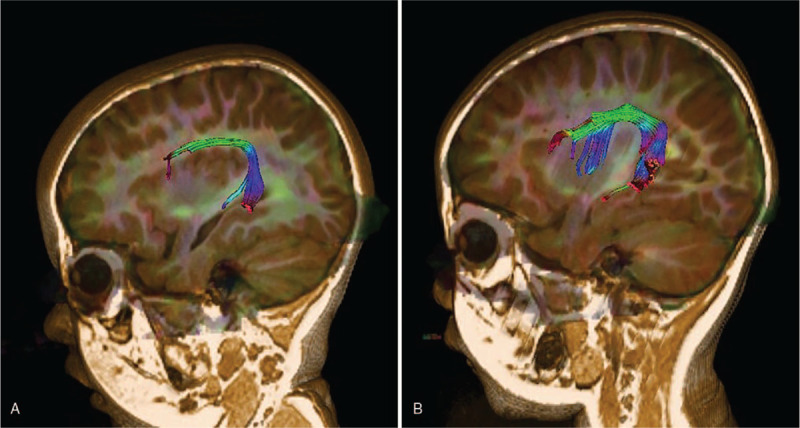
Three-dimensional (3D) tract reconstruction of the AF superimposed on sagittal T1 volume: (A) AF of a toddler with autism spectrum disorder with language delay; (B) AF of a toddler with developmental language disorder. AF = arcuate fasciculus.
Statistical analysis was performed using IBM SPSS v22 statistical software. Pearson's Chi-square test and independent sample t tests were used to compare gender, age, and IQ, and expressive language (EL) and receptive language (RL) scores, respectively. Inter-group differences in the DTI parameters of AF were analyzed using analysis of variance (ANOVA). We considered P values <.05 to indicate a significant difference.
4. Results
After T2WI acquisition, 1 toddler diagnosed with ASD who had gray matter heterotopias and 1 toddler with leukomalacia were excluded from the DLD group. In addition, 1 DLD toddler was excluded owing to failure to fuse images between T1 3D MP-RAGE and DTI. Finally, 15 ASD toddlers (11 males, 4 females; mean age 2.40 ± 0.60 years, range 1.42–3.25 years) and 16 DLD toddlers (10 males, 6 females; mean age 2.46 ± 0.67 years, range 1.25–3.83 years) were included.
As shown in Table 1, Pearson's Chi-square tests and independent sample t tests revealed no significant differences in gender, age or verbal IQ between the 2 groups. The ABC score of ASD group was significantly higher than that in DLD group (P <.05). However, the DLD group exhibited higher scores than the ASD group in terms of performance IQ, EL, and RL. As shown in Table 2, the ANOVA revealed that the ASD group exhibited significantly lower FA values (F = 8.560, P <.05), as well as significantly higher TV values (F = 8.158, P = .008) and NV values (F = 12.00, P = .002) compared with the DLD group. With age as the covariate, an analysis of covariance revealed significance differences in TV (F = 10.373, P = .003) and NV (F = 14.84, P = .001). ANOVA for AFL revealed no significant differences between the 2 groups (F = 2.158, P >.05).
Table 1.
Participant characteristics for autism spectrum disorder (ASD) and developmental language disorders (DLD).
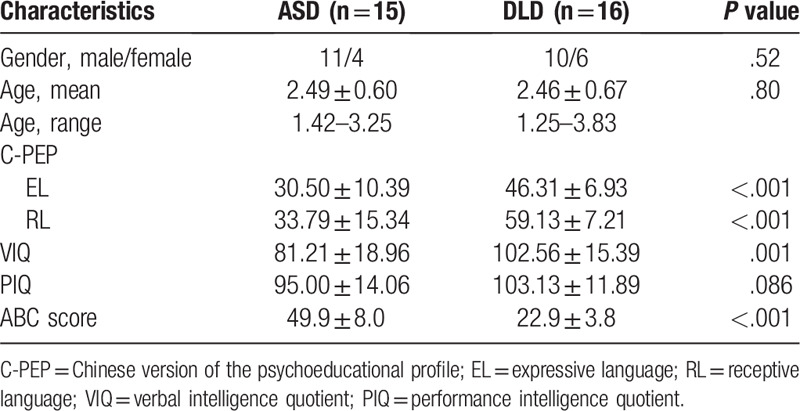
Table 2.
Diffusion tensor imaging parameters of arcuate fasciculus comparison between autism spectrum disorders (ASD) and developmental language disorders (DLD).
5. Discussion
Autism and related pervasive developmental disorders PDDs have typical neuropsychiatric characteristics, including impaired social interaction and communication skills, as well as repetitive behaviors.[12] Approximately 20/10,000 children are affected by ASD, and early symptoms can be identified from 1 to 3 years of age.[13]
Prominent theories of the mechanisms underlying ASD include impeded plasticity, excitation and inhibition dysregulation, mirror neurons, and theory of mind (ToM). The impeded plasticity theory proposes that both hypo- and hyper-connectivity exist in the brains of ASD children.[14,15] Similar to this theory, Frith and Happe hypothesized the involvement of “weak central coherence” in ASD, implicating impairments in the connections between different brain regions in the disorder.[6] ASD children have no organic problems in listening and speaking, but they do not respond normally to the information they hear, which is consistent with this hypothesis. White matter tracts play a critical role in neural connectivity. Disruption of the long-range white matter tracts that mediate connectivity within systems may be an important pathogenic factor contributing to core social impairments in ASD. Language delay is not the most common symptom of ASD but is the most common reason for hospitalization.[16,17] In the present study, we focused on the AF, a language-related white matter tract that has been studied since the 19th century, which connects the frontal expressive language area with the posterior temporoparietal receptive language areas.[18]
DTI can be used to examine both the macrostructure and microstructure of white matter tracts noninvasively in the brain, enabling assessment of the AF separately with the method described by Wakana et al[10] using both a seed and target ROI. Although methods using DTI to study of ASD have developed rapidly in the past decade, the early stages of ASD have received relatively little attention from DTI studies. Given the importance of early intervention in ASD, efficient methods for early diagnosis could be valuable. The clinical manifestations of ASD with language delay in the early stages among toddlers are similar to those of developmental language disorder (DLD), and differentiation is typically not possible using clinical methods alone. The current results suggest the presence of considerable differences in several DTI parameters between ASD and DLD, which could be helpful for the diagnosis of ASD.
The present study revealed that the ASD group had significantly lower FA values compared with the DLD group, consistent with the majority of previous research on ASD. There are several possible reasons for decreased FA values, including decreased myelination, decreased axonal density (a decrease in the number of axons in the AF with an increase in intra-axonal space), decreased organization of fibers, and increased tortuosity,[19–23] all of which have been confirmed in animal experiments.[24,25] The current results also suggest that toddlers with ASD exhibited significantly higher TV values and NV values than those with DLD. There are several potential explanations for these findings. Previous evidence suggests that the elimination and formation of synapses is a continuous process.[26,27] Dysregulation of synaptic pruning is reported to be responsible for the high local connectivity and low long-range connectivity observed in the brains of people with ASD,[28–30] and this erroneous or invalid connectivity may be involved in the neural mechanisms underlying higher TV and NV values. There is currently no clear consensus regarding this issue, despite the abundance of research on white matter tracts with DTI in recent decades. In addition to different research objectives, methods, and MRI scanners, these different results may have been affected by the dynamic and interconnected development of the white matter across the lifespan. Several previous studies reported that brain growth in children with ASD, particularly in the first 2 to 4 years of life, differs from that of typically developing children.[29,31–34]
Once diagnosed, ASD children should receive individualized treatment. The most widely used treatment is comprehensive rehabilitation therapy. For example, the C-PEP scale can be used to develop an individualized training program. Then, long-term follow-up assessment should be performed and the training program should be timely adjusted according to the efficacy of rehabilitation training. In addition, a systematic review by Serafini G et al[35] suggests that repetitive transcranial magnetic stimulation can enhance cognitive performance in treatment-resistant depression by specifically stimulating a functional brain region associated with neuropsychiatric disease, for example, the left dorsolateral prefrontal cortex. That is to say, the interaction between brain functional regions can be enhanced by stimulation, which is consistent with the theoretical basis of this study.
The present study involved several limitations that should be considered. First, the number of subjects in our sample was small. Due to the small sample size, this paper only studied the role of DTI parameters in identifying ASD and DLD. The upper/lower limit of DTI parameters for quantitative diagnosis of ASD was not obtained. Second, the diagnostic scale we used was relatively simple, and ASD children with language retardation, language deviancy, as well as other types of language disorders were ignored. In addition, other cognitive functions were not taken into consideration and were not well controlled. Thus, future studies with larger samples and more comprehensive scales are needed. Further research should be conducted to determine the brain areas involved in social interactions and communication skills related to neural connectivity, including longitudinal studies of the developmental trajectory of neural microstructure in people with ASD.
6. Conclusion
The current results demonstrate that the DTI parameters of AF, an important language-related white matter tract, in ASD toddlers with language delay are different from those in DLD toddlers, which are valuable for differentiating ASD with language delay from DLD among toddlers. DTI may provide an objective and effective method to aid clinical diagnosis and therapy in the early stages of ASD, and improve prognosis. However, further study with larger sample size is needed to determine the upper/lower limit of DTI parameters and to make quantitative diagnosis of ASD.
Acknowledgments
The authors thank the patients that contributed to this study. The authors also thank the Jining Rehabilitation Center for Autism for providing the patients’ materials. The authors thank Benjamin Knight, MSc, from Liwen Bianji, Edanz Editing China, for editing the English text of a draft of this manuscript.
Author contributions
KL and LZ were responsible for the study's design. RM contributed to the acquisition of neuroimaging sequences. YY and DL conducted the clinical data collection. LZ wrote the first draft of the manuscript. LZ, XLQ, and NZ analyzed the neuroimaging data. ZL and KL were responsible for statistical analysis. KL assisted in revising the overall manuscript. All authors read and approved the final manuscript.
Conceptualization: Lin Zhang, Kailong Li.
Data curation: Rui Ma, Xianlong Qi, Ning Zheng.
Investigation: Dandan Lian.
Methodology: Yanran Yuan.
Writing – original draft: Lin Zhang.
Writing – review & editing: Kailong Li.
Footnotes
Abbreviations: ASD = autism spectrum disorder, 3D MP-RAGE = 3-dimensional magnetization-prepared rapid acquisition gradient-echo, AFL = average fiber length, DLD = developmental language disorder, DTI = diffusion tensor imaging, FA = fractional anisotropy, NV = number of voxels, T2WI = T2-weighted imaging, TV = tract volume.
The authors have no conflicts of interest to disclose.
References
- [1].American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th Edition. Arlingtion: American Psychiatric Publishing, 2013: 50–59. [Google Scholar]
- [2].Cubells JF. Prevalence of autism spectrum disorders in China. Shanghai Arch Psychiatry 2013;25:176–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baron-Cohen S, Scott FJ, Allison C, et al. Prevalence of autism-spectrum conditions: UK school-based population study. Br J Psychiatry 2009;194:500–9. [DOI] [PubMed] [Google Scholar]
- [4].Hu VW, Steinberg ME. Novel clustering of items from the autism diagnostic interview-revised to define phenotypes within autism spectrum disorders. Autism Res 2009;2:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chlebowski C, Green JA, Barton ML, et al. Using the childhood autism rating scale to diagnose autism spectrum disorders. Autism Dev Disord 2010;40:787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Frith U, Happé F. Autism: beyond ‘theory of mind’. Cognition 1994;50:115–32. [DOI] [PubMed] [Google Scholar]
- [7].Pardini M, Garaci FG, Bonzano L, et al. White matter reduced streamline coherence in young men with autism and mental retardation. Eur J Neurol 2009;16:1185–90. [DOI] [PubMed] [Google Scholar]
- [8].Roine U, Roine T, Salmi J. Increased coherence of white matter fiber tract organization in adults with Asperger syndrome: a diffusion tensor imaging study. Autism Res 2013;6:642–50. [DOI] [PubMed] [Google Scholar]
- [9].Ameis SH, Catani M. Altered white matter connectivity as a nerual substrate for socialimpairment in Autism Spectrum Disorder. Cortex 2015;62:158–81. [DOI] [PubMed] [Google Scholar]
- [10].Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 2007;36:630–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Smits M, Jiskoot LC, Papma JM. White matter tracts of speech and language. Semin Ultrasound CT MR 2014;35:504–16. [DOI] [PubMed] [Google Scholar]
- [12].Washington DC. Diagnostic and Statistical Mental Disorders. 5rd ed. American Psychiatric Association 2013. [Google Scholar]
- [13].Newschaffer CJ, Croen LA, Daniels J, et al. The epidemiology of autism spectrum disorders. Annu Rev Public Health 2007;28:235–58. [DOI] [PubMed] [Google Scholar]
- [14].Kana RK, Uddin LQ, Kenet T, et al. Brain connectivity in autism. Front Hum Neurosci 2014;8:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Muller RA, Shih P, Keehn B, et al. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex 2011;21:2233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Groen WB, Zwiers MP, van der Gaag RJ. The phenotype and neural correlates of language in autism: an integrative review. Neurosci Biobehav Rev 2008;32:1416–25. [DOI] [PubMed] [Google Scholar]
- [17].Naigles LR. Input and language development in children with autism. Semin Speech Lang 2013;34:237–48. [DOI] [PubMed] [Google Scholar]
- [18].Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex 2008;44:953–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Alexander AL, Lee JE, Lazar M, et al. Diffusion tensor imaging of the brain. Neurotherapeutics 2007;4:316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Thomas C, Humphreys K, Jung KJ, et al. The anatomy of the callosal and visual-association pathways in high-functioning autism: a DTI tractography study. Cortex 2011;47:863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kumar A, Sundaram SK, Sivaswamy L, et al. Alterations in frontal lobe tracts and corpus callosum in young children with autism spectrum disorder. Cerebral Cortex 2010;20:2103–13. [DOI] [PubMed] [Google Scholar]
- [22].Jeong JW, Kumar AK, Sundaram SK, et al. Sharp curvature of frontal lobe white matter pathways in children with autism spectrum disorders: tract-based morphometry analysis. AJNR Am J Neuroradiol 2011;32:1600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Harsan LA, Poulet, Guignard B, et al. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J Neurosci Res 2006;83:392–402. [DOI] [PubMed] [Google Scholar]
- [24].Tyszka JM, Readhead C, Bearer EL, et al. Statistical diffusion tensor histology reveals regional dysmyelination effects in the shiverer mouse mutant. Neuroimage 2006;29:1058–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 2005;26:132–40. [DOI] [PubMed] [Google Scholar]
- [26].Keehn B, Wagner JB, Tager-Flusberg H, et al. Functional connectivity in the first year of life in infants at-risk for autism: a preliminary near-infrared spectroscopy study. Front Hum Neurosci 2013;7:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Assaf M, Jagannathan K, Calhoun VD, et al. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. NeuroImage 2010;53:247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Stiles J. The fundamentals of brain development. Cambridge MA: Harvard University Press; 2008. [Google Scholar]
- [29].Schumann CM, Bloss CS, Carter Barnes C, et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci 2010;30:4419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Thomas MSC, Johnson MH. New advances in understanding sensitive periods in brain development. Curr Dir Psychol Sci 2008;17:1–5. [Google Scholar]
- [31].Bloss CS, Courchesne E. MRI neuroanatomy in young girls with autism: a preliminary study. J Am Acad Child Adolesc Psychiatry 2007;46:515–23. [DOI] [PubMed] [Google Scholar]
- [32].Nordahl CW, Lange N, Li DD, et al. Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proc Natl Acad Sci U S A 2011;108:20195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Courchesne E, Mouton PR, Calhoun ME, et al. Neuron number and size in prefrontal cortex of children with autism. JAMA 2011;306:2001–10. [DOI] [PubMed] [Google Scholar]
- [34].Wolff JJ, Gu H, Gerig G, et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry 2012;169:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Serafini G, Pompili M, Belvederi Murri M, et al. The effects of repetitive transcranial magnetic stimulation on cognitive performance in treatment-resistant depression. A systematic review. Neuropsychobiology 2015;71:125–39. [DOI] [PubMed] [Google Scholar]


