Abstract
Background:
Soluble mesothelin-related peptide (SMRP) is a widely studied tumor marker for diagnosing malignant pleural mesothelioma (MPM). This study discussed the diagnostic value of SMRPs in pleural effusion (PE) for MPM.
Methods:
Medline, Embase, Web of Science, and Cochrane library system were systematically searched on the data of SMRPs in PE for MPM diagnosis. Pooled diagnostic sensitivity, specificity, and symmetric receiver operating characteristic curve were calculated.
Results:
Thirteen studies fulfilled the inclusion criteria and a total of 3359 cases including 759 MPM cases, 1061 non-MM (malignant mesothelioma) malignant PE, and 1539 benign PE were brought into this meta-analysis. The pooled results of SMRPs in PE for diagnosing MPM were as follows: sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio were 0.68 (95% confidence interval [CI]: 0.64–0.72), 0.91 (95% CI: 0.86–0.94), 7.8 (95% CI: 5.0–12.0), 0.35 (95% CI: 0.31–0.40), and 22 (95% CI: 14–35), respectively. The area under the summary receiver operating characteristic curves (AUC) was 0.75 (95% CI: 0.72–0.80). Subgroup analyzes revealed that the AUC of cohort group using histological diagnosis could be improved to 0.86 (95% CI: 0.83, 0.89). The Deek's funnel plot asymmetry test showed no publication bias.
Conclusion:
Although the sensitivity of SMRPs was low, PE-SMRPs can be a good indicator of the existence of MPM.
Keywords: accuracy, diagnosis, mesothelin, mesothelioma, pleural effusion
1. Introduction
Malignant pleural mesothelioma (MPM) is a rapidly developing, almost fatal malignant tumor.[1] Although its occurrence is relatively rare, it is mainly associated with exposure to asbestos. Because the carcinogenicity of asbestos has been detected in recent years, it has been strictly prohibited in the United States, Europe, Australia, and so on.[2] However, in some industrialized countries, such as China, the production and use of asbestos are still not subject to strict control, which conferring the incidence of MPM will continue to increase in the coming decades.[3,4] A large study shows that the global MPM death burden is assumed to be about 38,400 a year, probably well above that.[5] However, due to the lack of a history of asbestos exposure and difficulty in obtaining pathological tissue for examination, the diagnosis of mesothelioma may be underestimated.[6] Exploring biomarkers of mesothelioma is helpful for screening and early diagnosis of mesothelioma and improving prognosis.
In diagnosis, the most widely studied biomarkers are mesothelin, osteopontin, fibulin-3, and high mobility group box protein 1.[7–10] Of which, soluble mesothelin-related peptides (SMRPs) is one of the most robust biomarkers at present for the diagnosis of MPM. Mesothelin is a 40-kD cell surface glycoprotein, which has putative functions in cell-to-cell adhesion.[11] It is highly overexpressed in cancers such as malignant mesothelioma, pancreatic or ovarian carcinoma, sarcomas, and in some gastrointestinal or pulmonary carcinomas.[12] Through 2 mechanisms of abnormal splicing events or an enzymatic cleavage from membrane-bound mesothelin, elevated amounts of SMRPs could be found in pleural effusions (PE).[13] This provides the possibility for the application of PE-SMRPs in the diagnosis of MPM.
Actually, the diagnostic accuracy of SMRPs for MPM has been extensively studied, previous diagnostic experiments have shown that PE-SMRPs has higher specificity and good diagnostic efficacy.[14] In particular, previous meta-analysis studies on SMRPs in blood and PE showed certain diagnostic value.[7,15] Considering the small number of meta-analysis by Cui et al, we performed a meta-analysis to update the overall diagnostic accuracies of SMRPs in PE for identifying MPM[7].
2. Methods
Neither ethical approval nor consent to participate is applicable because this is a meta-analysis based on previous published studies.
2.1. Publication search strategy
The electric database included Medline, Embase, Web of Science, and Cochrane library, were systematically searched until August 24, 2018. The search keywords were as follow: “mesothelin” or “soluble mesothelin-related peptides” or “SMRPs” and “malignant pleural mesothelioma” or “mesothelioma” and “diagnosis” or “diagnostic value” or “diagnostic accuracy”. Studies were included when it provided diagnostic value of SMRPs in PE with both sensitivity and specificity for diagnosing MPM from other diseases included non-MPM malignancy, benign and undiagnosed disease.
2.2. Inclusion and exclusion criteria
Participants with PE were identified for inclusion. The diagnosis of MPM was proven on histopathology obtained by biopsy histopathology or pleural fluid cytology.
The exclusion criteria were: reviews, meta-analyses, case reports, letters, or expert opinions; SMRPs detected in other specimens, not in PE; studies published in languages other than English or Chinese; insufficient data to calculate sensitivity and specificity; duplicates; multiple publications.
2.3. Data extraction and risk of bias assessment
Two reviewers (R.G., Q.Y.) independently extracted data from included trials and assessed the risk of bias. The extracted data mainly included 9 aspects: the first author; the year of the paper published; the region the study performed; the study type; the measurement of SMRPs; the standard criteria of MPM; the number of all included patients; the number of MPM patients; the number of true positive, false positive, false negative, and true negative. The risk of bias of the included studies was assessed by the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) tool and rated as “low,” “unclear,” or “high” in several domains. The two reviewers search and evaluate the quality of the studies independently. Any disagreement was resolved by a third reviewer (F.W.).
2.4. Statistical analyses
The diagnostic efficacy for PE-SMRPs was pooled by the equation of sensitivity = true positive/ (true positive + false negative), specificity = true negative/(true negative + false positive). The diagnostic parameters were pooled by the random effect model according to the statistical heterogeneity across the included studies. The symmetric receiver operating characteristic curve (SROC) was drawn and area under the curve (AUC) was calculated through sensitivity vs. specificity for diagnosing MPM. P < .05 was considered statistically significant. Subgroup analysis was used to explore sources of heterogeneity. The subgroup analysis was carried out according to the risk of bias on patient selection, index test, reference standard, flow, and timing. Statistical analyses were conducted using Review Manager Version 5.2 (Cochrane IMS, Oxford, UK), Stata version 15.0 (Stata Corporation, College Station, TX).
3. Results
This study was confirmed by the PROSPERO register (Registration ID CRD42018116506). We identified 349 studies, from which 123 were duplicates, showed in Figure 1. After screening titles and abstracts, 188 trials were excluded because of not meeting the inclusion criteria. Then, we obtained full-text articles for 38 citations, of which, 25 were excluded for reasons described in the Figure 1. Ultimately, 13 studies met our eligibility criteria were included in this meta-analysis, involving a total of 3359 patients.[16–28] One study had 2 best diagnostic performances, which were considered 2 independent studies.[17]
Figure 1.
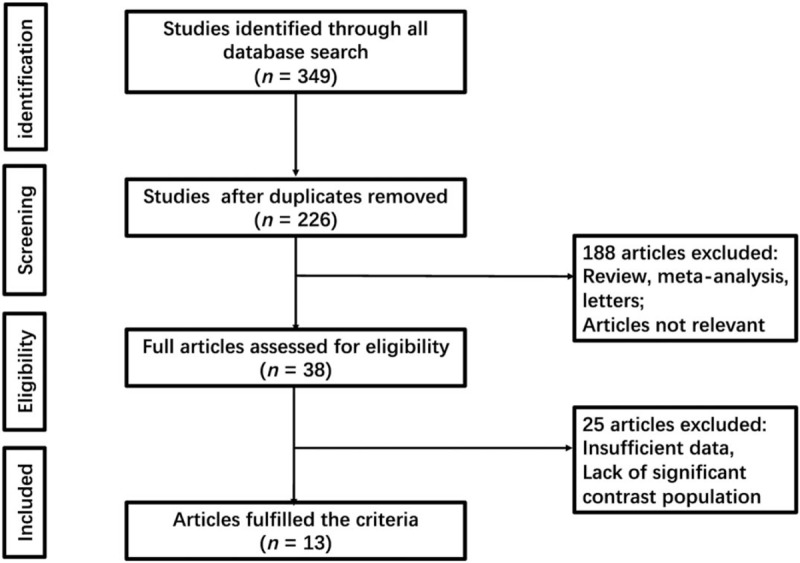
Flow chart of selection process in this study.
3.1. The characteristic of included studies
The included studies were published between 2007 and 2017. The average sample size was 240 (range from 92 to 1331) and the total of 3356 patients was included, of whom, 756 were MPM patients, 1061 were non-MPM malignant PE cases, and 1539 were benign PE cases. The characteristic of included studies was presented in Table 1.
Table 1.
The characteristic of included 13 studies.
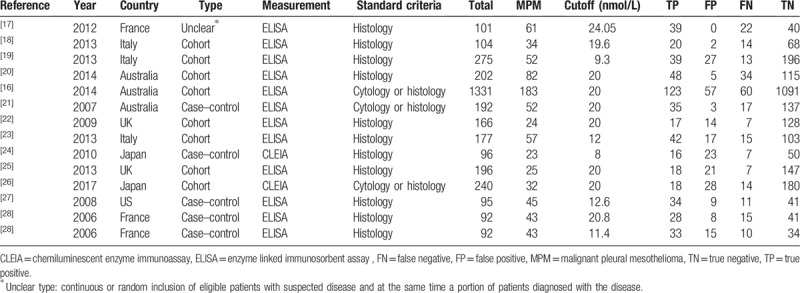
Most studies[17–26] used histopathologic immunochemistry method as diagnostic criteria for MPM, only 3 studies[16,27,28] used PE cytology with biopsy histology as diagnostic criteria.
In term of the type of study, 8 studies[16,19–23,25,28] were cohort study that enrolled a consecutive or random sample of patients, 4 studies[17,24,26,27] were case–control designed, and 1 study[18] was an unclear type that enrolled a consecutive sample of eligible patients with suspected disease and a portion of participants with known disease. In addition to 2 studies[24,28] measuring SMRPs using the chemiluminescent enzyme immunoassay method, the rest of the studies used enzyme linked immunosorbent assay method. Finally, the cutoff values applied in 4 articles[16,21,25,28] were previously established.
3.2. Risk of bias
The assessment of risk of bias was presented in Figure 2, which indicated patient selection, and reference standard existed high risk on the bias. Five studies[17,18,24,26,27] had a high risk of bias on patient selection because these studies were not a consecutive sample of patients enrolled. One study[28] had an unclear risk of bias on patient selection because it was a retrospective study. Three studies[16,27,28] had a high risk of bias on reference standard because these studies used pleural fluid cytology as diagnostic criteria, which remain a controversial subject according to guideline.[6] Nine studies[17–20,22–24,26,27] had an unclear risk of bias on index text because these studies selected the test threshold to optimize sensitivity for evaluating diagnostic effectiveness, however, this bias might be moderate risk for eligible sample sizes.[29]
Figure 2.
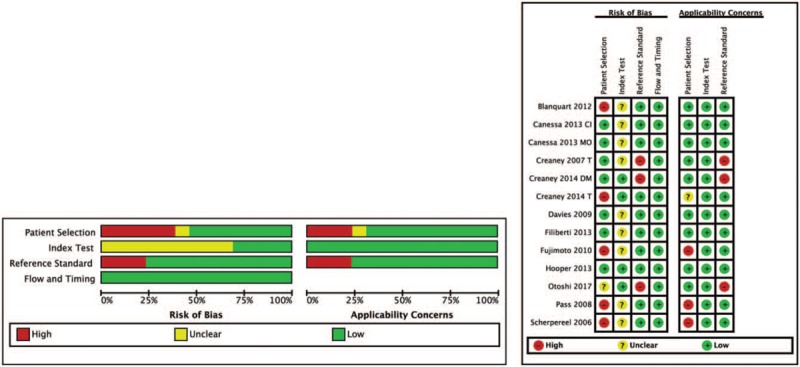
The general quality of the included 13 studies. Adapted with permission from[16,19,20,21,27].
3.3. Diagnostic accuracy
We noted that sensitivity was 0.69 (95% confidence interval [CI]: 0.64 to 0.72), specificity was 0.90 (95% CI: 0.85–0.94), positive likelihood ratio (PLR) was 4.78 (95% CI: 3.52–6.50), negative likelihood ratio (NLR) was 0.30 (95% CI: 0.24–0.36), and diagnostic odds ratio (DOR) was 19.50 (95% CI: 12.14–31.33). A forest plot of sensitivity and specificity for 13 studies of PE SMRPs for the diagnosis of MPM was presented in Figure 3. I2 values of sensitivity and specificity were 2.97 (P = .42), 89.88 (P < .001), respectively, indicating massive heterogeneity in specificity between studies.
Figure 3.
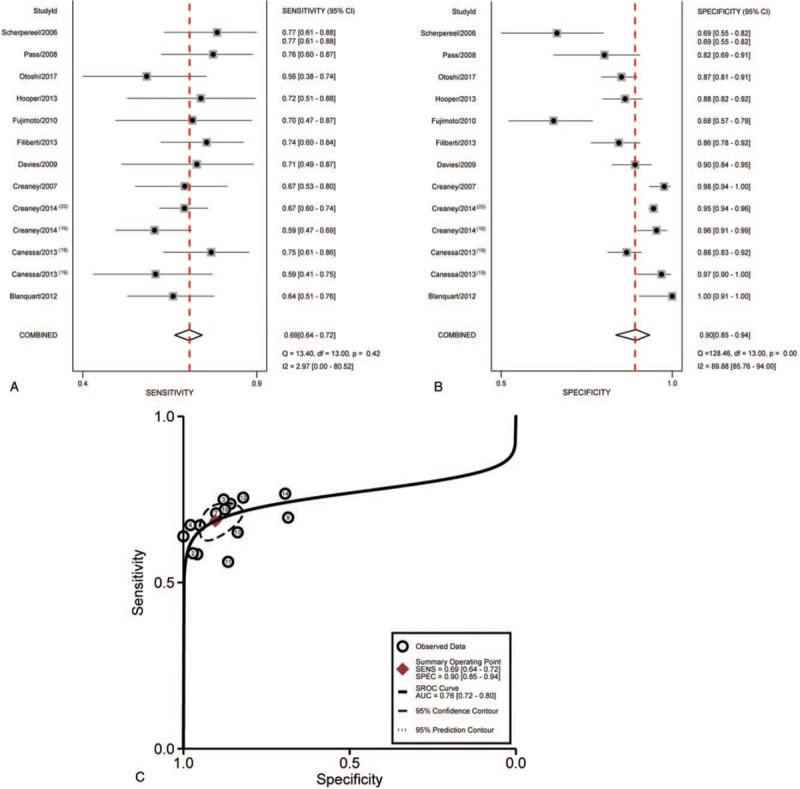
Forest plot of estimates of sensitivity (A) and specificity (B) for SMRPs in pleural fluid in the diagnosis of malignant mesothelioma and Summary receiver operating characteristic curve (C) for SMRPs which summarize the overall diagnostic accuracy. Each circle represents each study in the meta-analysis. SMRPs = soluble mesothelin-related peptides.
A graph of SROC for the SMRPs differentiates MPM from other diseases was shown in Figure 3. The AUC was 0.75 (95% CI: 0.72–0.80), indicating the level of overall accuracy was not as high as expected.
3.4. Subgroup analysis
Preliminary analysis showed that the overall diagnostic ability of PE-SMRPs was not as good as predicted with high heterogeneity. According to quality assessment, patient selection, the applicability of diagnostic criteria, and presupposition of cutoff value might be the cause of high heterogeneity.
For further discover the diagnostic value of PE-SMRPs, first of all, we divided the literature into 2 groups, one was a cohort study group and the other was the noncohort study group, and remove studies by PE cytological diagnosis. Unfortunately, the removal of 1 study of PE criteria from the noncohort group could not be analyzed by Stata for asymmetric data, so the 1 study was not removed.[27] The diagnostic comparison of sensitivity, specificity, PLR, NLR, DOR, and AUC between cohort group and noncohort group were showed in Table 2. The forest plot and the SROC of the cohort group and noncohort group were showed Figure 4, respectively.
Table 2.
The comparison of diagnostic performance between cohort group and non-cohort group.
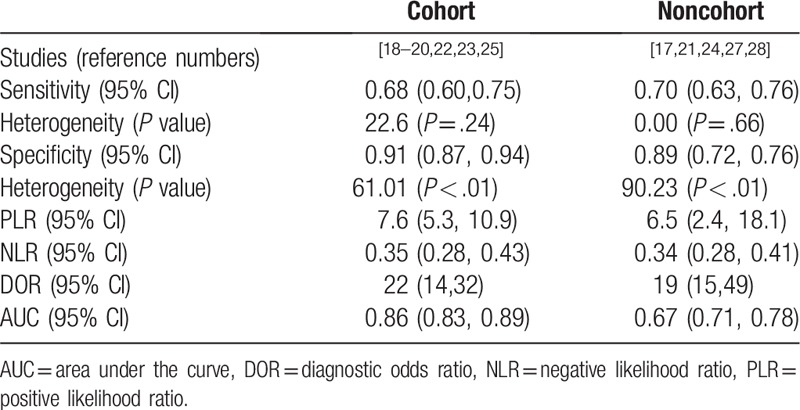
Figure 4.
Forest plot of estimates of sensitivity and specificity and summary receiver operating characteristic curve for SMRPs in the cohort group and noncohort group. SMRPs = soluble mesothelin-related peptides.
The PE-SMRPs in cohort group had better diagnostic value and lower heterogeneity compared with the noncohort group, the AUC of the SROC of the cohort group was 0.86 with pooled sensitivity of 0.68 (95% CI: 0.60–0.75) and the pooled specificity of 0.91 (95% CI: 0.87–0.94), which showed in Figure 3. However, there might be a medium specificity due to the absence of analysis of the influence of preset cutoff.
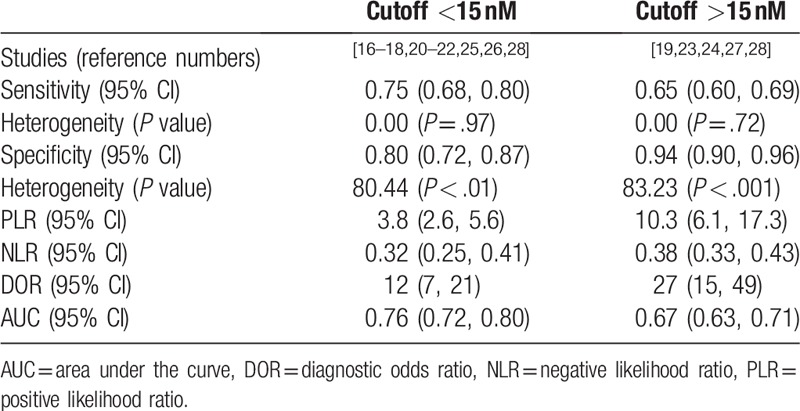
We divided the study into 2 groups according to whether the cutoff value was greater than or less than 15 nM. However, the results could not reduce the heterogeneity which indicated meaningless. The results were shown in the Table 3.
Table 3.
The comparison of diagnostic performance between cutoff < 15 nM and non-cutoff >15 nM.
3.5. Publication bias
The Deek's test showed P = .13, which revealed there was no publication bias in this meta-analysis, showed in Figure 5.
Figure 5.
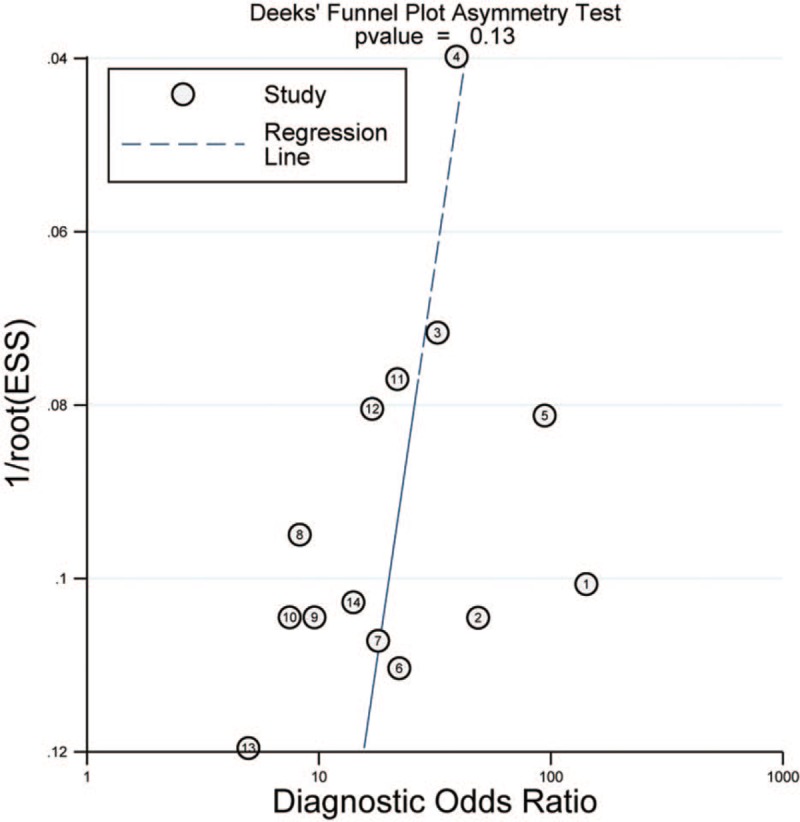
The Deek's funnel plot of publication bias for SMRP in diagnosis of MPM. MPM = malignant pleural mesothelioma, SMRPs = soluble mesothelin-related peptides.
4. Discussion
Since the abundant application of asbestos in the 1960s, the incidence of MPM is increasing. For clinicians, MPM is still susceptible to miss due to its low morbidity and nonspecific clinical manifestations. Moreover, the most recommended approach to diagnose MPM necessitates invasive examination such as thoracoscopy, which is restricted to achieve for old patients and increased the difficulty for diagnosis. It was worth noting that carcinoembryonic antigen (CEA) is a very widely used tumor marker, and PE-CEA had a superior diagnostic value in the diagnosis of malignant PE,[30] but its diagnostic value for MPM was low.[31] So far, the diagnosis of MPM depends entirely on histopathological examination, without a single biological marker, which is unfavorable for early diagnosis. SMRPs is one of the most popular soluble biomarker among studies.[6] Given fewer patients taking into account previous meta-analysis, we therefore, performed a meta-analysis to update the overall diagnostic accuracies of pleural SMRPs.[32]
Our results suggest that PE-SMRPs had an unfavorable diagnostic performance with poor sensitivity (0.69 95% CI: 0.64, 0.72) and high specificity (0.90 95% CI: 0.85, 0.94). The AUC was 0.76 (95% CI: 0.72, 0.80) indicating that the overall accuracy was not as high as expected. On one hand, high specificity revealed that PE-SMRPs above the cutoff value could be helpful for confirming MPM, so further invasive examination, will be strongly supported. On the other hand, low sensitivity (0.69 95% CI: 0.64, 0.72) limited SMRPs’ diagnostic performance. When the level of SMRPs was lower than cutoff value, the diagnosis could not exclude from MPM. In general, the PLR is greater than 10 and the NLR is less than 0.1 to exclude the possibility of disease.[33] This indicates that PE-SMRPs does not have the ability to diagnose MPM directly, but it also has certain diagnostic value. In view of the heterogeneity of specificity (I2 = 89.88, P < .01), this reveal the existence of inclusion bias, so further subgroup analysis is requisite.
The QUADAS tool is the widest tool to assess the quality of included studies in systematic reviews of diagnostic accuracy studies for markedly heterogeneous results, which is improved to QUADAS-2.[34,35] The results of QUADAS-2 (Fig. 1) suggested patient selection, the applicability of diagnostic criteria, and cutoff value might be the main cause of heterogeneity. Among them, the type of study and diagnostic criteria has a greater impact on the bias. The presence of an inappropriate exclusion of undiagnosed PE in the case–control study might result in overestimation of diagnostic accuracy.[36] At the same time, diagnosis in cytology remains controversial because the sensitivity of cytology for the diagnosis of mesothelioma ranges from 30% to 75% and epithelioid mesothelial cells are easier to exfoliate tumor cells in the pleural cavity compared with sarcomatoid mesothelioma, which misleads the pathologist and reduces nonepithelioid mesothelioma diagnosis.[37] To sum up, the cytology diagnosis is unreliable.
According to the type of study, 13 studies were divided into cohort study group and noncohort study group. It was worth noting that the study of cytological diagnosis was excluded in the cohort group but not in the noncohort group for calculating. The result showed cohort group provided better diagnostic accuracy than the noncohort group for MPM: AUC was 0.86 (95% CI: 0.83–0.89) compared with 0.76 (95% CI: 0.71–0.78), meanwhile, the heterogeneity of specificity in cohort group decline to 61.01 (P < .01). Therefore, we believe that the results of the cohort group can better reflect the actual diagnostic value of SMRPs.
However, the results of the cohort group also had low sensitivity, similar to previous study results, which might be related to its production.[7] SMRPs is a glycoprotein on the surface of epithelial cells. The production of SMRPs is related to abnormal splicing events leading to the synthesis of a secreted protein or to an enzymatic cleavage from membrane-bound mesothelin, which could be found in PE.[13] This characteristic might be the main reason for its low sensitivity of SMRPs for MPM. In the next place, SMRPs is most expressed in epithelial mesothelioma, while almost absent in sarcomatous type. When the proportion of sarcomatous or other types of mesothelioma is increased, combined with cytology diagnosis, it leads to increased false negative rate and reduced sensitivity. Moreover, SMRPs content is less in the early stage of mesothelioma, so more cases of early (I II period) are included, which will also lead to poor sensitivity. Unfortunately, our study did not make a further assessment of the diagnostic effectiveness of SMRPs by stages.
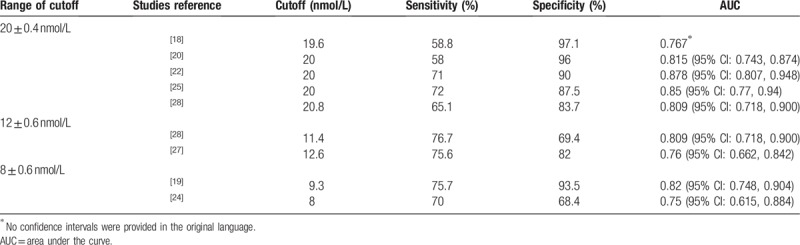
As a meta-analysis, the cutoff values of the articles included in this study are quite different (from minimum 8 nmol/L to maximum 24.1 nmol/L). Therefore, with 15 nmol/L as the boundary, we are divided included studies into 2 groups according to the cutoff values, which were presented in Table 3. The results showed that group (cutoff <1 5 nM) had higher diagnostic efficacy because it had higher AUC (0.76 (95% CI: 0.72, 0.80) vs. 0.67 (95% CI: 0.63, 0.71)), but lower specificity (0.80 [95% CI: 0.72, 0.87] vs. 0.94 [95% CI: 0.90, 0.96]) and lower PLR (3.8 [95% CI: 2.6, 5.6] vs. 10.3 [95% CI: 6.1, 17.3]), which meant that more suspicious MPM patients could be included in context of lower level of cutoff value of PE-SMRPS with a compromised ability to confirm MPM. In addition, we also compared the diagnostic effectiveness of actual experiments with different cutoff values, which showed in Table 4. The results revealed that AUC maintained from 0.767 to 0.878 with cutoff value (20 ± 0.4 nmol/L). Moreover, the specificity with cutoff value of 20 ± 0.4 nmol/L was generally higher than that of cutoff value of 12 ± 0.6 nmol/L and 8 ± 0.6 nmol/L, with a range of 83.7% to 97.1%. Altogether, we can conclude that MPM should be suspected when PE-SMRPS >8 nmol/L. When PE-SMRPS >20 nmol/L, MPM should be strongly suspected and further invasive procedures may be intended for patients.
Table 4.
Diagnostic effectiveness of different threshold ranges.
There are several advantages to this study. First, we assessed the diagnostic performance of PE-SMRPs differentiating other diseases from MPM through subgroup analysis, which was helpful for application practice. Furthermore, our results were consistent with those of Cui et al, we involved a larger number of patients and the subgroup analysis of the type was carried out, which had more reference value.[7]
However, this study also presents several limitations. First of all, only full English studies included in the present study and exclusion of conference abstracts and non-English language studies might result in publication bias. However, the results of Deek's test showed that there was no publication bias in this study. Next, the subgroup analysis could not be carried out by meta-regression analysis, this was due to the cause that Stata could not be calculated for asymmetric data. Nonetheless, our results according to the consequence of QUADAS-2 showed that the direction of subgroup analysis was correct, which effectively reduced the heterogeneity and improved the diagnostic efficacy.
Ultimately, SMRPs in PE can be a good indicator of the existence of MPM. Though poor sensitivity of SMRPs might miss sarcomatous or another type mesothelioma, high specificity of SMRPs could point out mesothelioma, which provides strong evidence for further invasive examination. It is necessary to further explore highly sensitive biomarkers combined with SMRPs for the diagnosis of MPM.
5. Conclusion
SMRPs in PE can be a good indicator of the existence of MPM, high level of pleural SMRPs are highly suspicious for MPM which provides supporting evidence for further invasive examinations to obtain biopsy results from the pleura. However, its sensitivity is too low to be used as an indicator of MPM screening.
Author contributions
Ruiyue Gao, Yue Qin collected the relevant papers and data. Ruiyue Gao performed statistical analysis and data synthesis. All authors contributed substantially to the study design, data interpretation, and the writing of the manuscript. Feng Wang gave critical comments and revised the paper. All authors approved the final version of the manuscript.
Conceptualization: Yanbing Wu.
Data curation: Yue Qin.
Formal analysis: Ruiyue Gao.
Funding acquisition: Lili Xu.
Project administration: Lili Xu.
Resources: Zhen Wang.
Software: Feng Wang.
Supervision: Huanzhong Shi, Zhaohui Tong.
Visualization: Feng Wang.
Writing – original draft: Ruiyue Gao.
Writing – review & editing: Feng Wang, Zhen Wang.
Ruiyue Gao orcid: 0000-0001-6664-259X.
Footnotes
Abbreviations: AUC = area under the curve, CEA = carcinoembryonic antigen, CEA = carcinoembryonic antigen, CLEIA = chemiluminescent enzyme immunoassay method, DOR = diagnostic odds ratio, ELISA = enzyme linked immunosorbent assay method, FN = false negative, FP = false positive, HGMB = high mobility group box protein 1, MM = malignant mesothelioma, MPM = malignant pleural mesothelioma, NLR = negative likelihood ratio, PE = Pleural effusions, PLR = positive likelihood ratio, QUADAS = Quality Assessment of Diagnostic Accuracy Studies, SMRPs = soluble mesothelin-related peptides, SROC = summary receiver operating characteristic, TN = true negative, TP = true positive.
Ruiyue Gao and Feng Wang contributed equally to this study.
This study was supported by the Beijing Municipal Administration of Hospital's Youth (No. QML20170304).
The authors have no conflicts of interest to disclose.
PROSPERO registration number: CRD42018116506.
References
- [1].McCambridge AJ, Napolitano A, Mansfield AS, et al. Progress in the management of malignant pleural mesothelioma in 2017. J Thorac Oncol 2018;13:606–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Baumann F, Ambrosi JP, Carbone M. Asbestos is not just asbestos: an unrecognised health hazard. Lancet Oncol 2013;14:576–8. [DOI] [PubMed] [Google Scholar]
- [3].Guo Z, Carbone M, Zhang X, et al. Improving the accuracy of mesothelioma diagnosis in China. J Thorac Oncol 2017;12:714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Leong SL, Zainudin R, Kazan-Allen L, et al. Asbestos in Asia. Respirology 2015;20:548–55. [DOI] [PubMed] [Google Scholar]
- [5].Odgerel CO, Takahashi K, Sorahan T, et al. Estimation of the global burden of mesothelioma deaths from incomplete national mortality data. Occup Environ Med 2017;74:851–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Woolhouse I, Bishop L, Darlison L, et al. British Thoracic Society Guideline for the investigation and management of malignant pleural mesothelioma. 2018; 1–30 [DOI] [PubMed] [Google Scholar]
- [7].Cui A, Jin XG, Zhai K, et al. Diagnostic values of soluble mesothelin-related peptides for malignant pleural mesothelioma: updated meta-analysis. BMJ Open 2014;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hu Z. De, Liu XF, Liu XC, et al. Diagnostic accuracy of osteopontin for malignant pleural mesothelioma: a systematic review and meta-analysis. Clin Chim Acta 2014;433:44–8. [DOI] [PubMed] [Google Scholar]
- [9].Pass HI, Levin SM, Harbut MR, et al. Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma. N Engl J Med 2012;367:1417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Napolitano A, Antoine DJ, Pellegrini L, et al. HMGB1 and its hyperacetylated isoform are sensitive and specific serum biomarkers to detect asbestos exposure and to identify mesothelioma patients. Clin Cancer Res 2016;22:3087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rump A, Morikawa Y, Tanaka M, et al. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem 2004;279:9190–8. [DOI] [PubMed] [Google Scholar]
- [12].Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci U S A 1996;93:136–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sapede C, Gauvrit A, Barbieux I, et al. Aberrant splicing and protease involvement in mesothelin release from epithelioid mesothelioma cells. Cancer Sci 2008;99:590–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yamada S, Tabata C, Tabata R, et al. Clinical significance of pleural effusion mesothelin in malignant pleural mesothelioma. Clin Chem Lab Med 2011;49:1721–6. [DOI] [PubMed] [Google Scholar]
- [15].Luo L, Shi HZ, Liang QL, et al. Diagnostic value of soluble mesothelin-related peptides for malignant mesothelioma: a meta-analysis. Respir Med 2010;104:149–56. [DOI] [PubMed] [Google Scholar]
- [16].Creaney J, Segal A, Olsen N, et al. Pleural fluid mesothelin as an adjunct to the diagnosis of pleural malignant mesothelioma. Dis Markers 2014;2014:413946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Scherpereel A, Grigoriu B, Conti M, et al. Soluble mesothelin-related peptides in the diagnosis of malignant pleural mesothelioma. Am J Respir Crit Care Med 2006;173:1155–60. [DOI] [PubMed] [Google Scholar]
- [18].Blanquart C, Gueugnon F, Nguyen MM, et al. CCL2, galectin-3, and SMRP combination improves the diagnosis of mesothelioma in pleural effusions. J Thorac Oncol 2012;7:883–9. [DOI] [PubMed] [Google Scholar]
- [19].Canessa PA, Ferro P, Manta C, et al. Clinical value of mesothelin in pleural effusions versus histology by medical thoracoscopy: brief report. Med Oncol 2013;30:649. [DOI] [PubMed] [Google Scholar]
- [20].Canessa PA, Franceschini MC, Ferro P, et al. Evaluation of soluble mesothelin-related peptide as a diagnostic marker of malignant pleural mesothelioma effusions: its contribution to cytology. Cancer Invest 2013;31:48–55. [DOI] [PubMed] [Google Scholar]
- [21].Creaney J, Dick IM, Meniawy TM, et al. Comparison of fibulin-3 and mesothelin as markers in malignant mesothelioma. Thorax 2014;69:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Davies HE, Sadler RS, Bielsa S, et al. Clinical impact and reliability of pleural fluid mesothelin in undiagnosed pleural effusions. Am J Respir Crit Care Med 2009;180:437–44. [DOI] [PubMed] [Google Scholar]
- [23].Filiberti R, Parodi S, Libener R, et al. Diagnostic value of mesothelin in pleural fluids: comparison with CYFRA 21-1 and CEA. Med Oncol 2013;30:543. [DOI] [PubMed] [Google Scholar]
- [24].Fujimoto N, Gemba K, Asano M, et al. Soluble mesothelin-related protein in pleural effusion from patients with malignant pleural mesothelioma. Exp Ther Med 2010;1:603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hooper CE, Morley AJ, Virgo P, et al. A prospective trial evaluating the role of mesothelin in undiagnosed pleural effusions. Eur Respir J 2013;41:18–24. [DOI] [PubMed] [Google Scholar]
- [26].Pass HI, Wali A, Tang N, et al. Soluble mesothelin-related peptide level elevation in mesothelioma serum and pleural effusions. Ann Thorac Surg 2008;85:265–72. [DOI] [PubMed] [Google Scholar]
- [27].Creaney J, Yeoman D, Naumoff LK, et al. Soluble mesothelin in effusions: a useful tool for the diagnosis of malignant mesothelioma. Thorax 2007;62:569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Otoshi T, Kataoka Y, Ikegaki S, et al. Pleural effusion biomarkers and computed tomography findings in diagnosing malignant pleural mesothelioma: a retrospective study in a single center. PLoS One 2017;12:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Leeflang MM, Moons KG, Reitsma JB, et al. Bias in sensitivity and specificity caused by data-driven selection of optimal cutoff values: mechanisms magnitude, and solutions. Clin Chem 2008;54:729–37. [DOI] [PubMed] [Google Scholar]
- [30].Yang Y, Liu YL, Shi HZ. Diagnostic accuracy of combinations of tumor markers for malignant pleural effusion: an updated meta-analysis. Respiration 2017;94:62–9. [DOI] [PubMed] [Google Scholar]
- [31].Zhai K, Wang W, Wang Y, et al. Diagnostic accuracy of tumor markers for malignant pleural effusion: a derivation and validation study. J Thorac Dis 2017;9:5220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Boudville N, Paul R, Robinson BWS, et al. Mesothelin and kidney function—analysis of relationship and implications for mesothelioma screening. Lung Cancer 2011;73:320–4. [DOI] [PubMed] [Google Scholar]
- [33].Jaeschke R, Guyatt G, Sackett DL. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA 1994;271:389–91. [DOI] [PubMed] [Google Scholar]
- [34].Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Willis BH, Quigley M. Uptake of newer methodological developments and the deployment of meta-analysis in diagnostic test research: a systematic review. BMC Med Res Methodol 2011;11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Whiting PF, Rutjes AW, Westwood ME, et al. Research and reporting methods accuracy studies. Ann Intern Med 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- [37].Cristaudo A, Bonotti A, Guglielmi G, et al. Serum mesothelin and other biomarkers: what have we learned in the last decade? J Thorac Dis 2018;10:S353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


