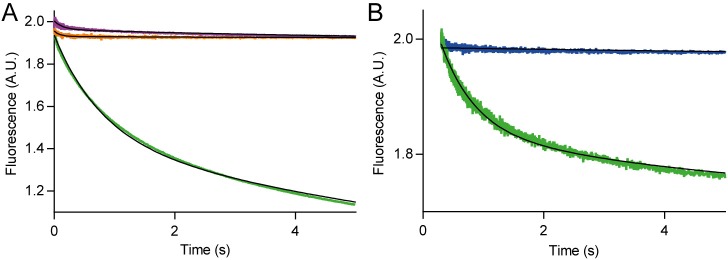
Fig 4. Kinetics of PfR6 interaction with HMG-box.
HMG-box540Q and PfR6FAM were used to monitor the interaction between the aptamer and the protein in real-time using a stopped-flow apparatus (Applied Photophysics, UK). (A) FRET between HMG-box540Q and PfR6FAM. Mixing the labeled protein with the probe-bound aptamer (green) resulted in a decrease of fluorescence over time, indicating that the binding of both components results in the quenching of fluorescein due to its vicinity with the quencher in the protein. FRET assessment controls lacking the aptamer or the quencher Atto-540 in the HMG-box are shown in orange and purple traces respectively. (B) HMG-box 540Q Pf (green) or HMG-box 540Q Hs (blue) comparison during the binding with PfR6FAM at pH 5.5. 7–10 replicates for each reaction were measured and averaged. Continuous lines show the fitting with exponential functions.