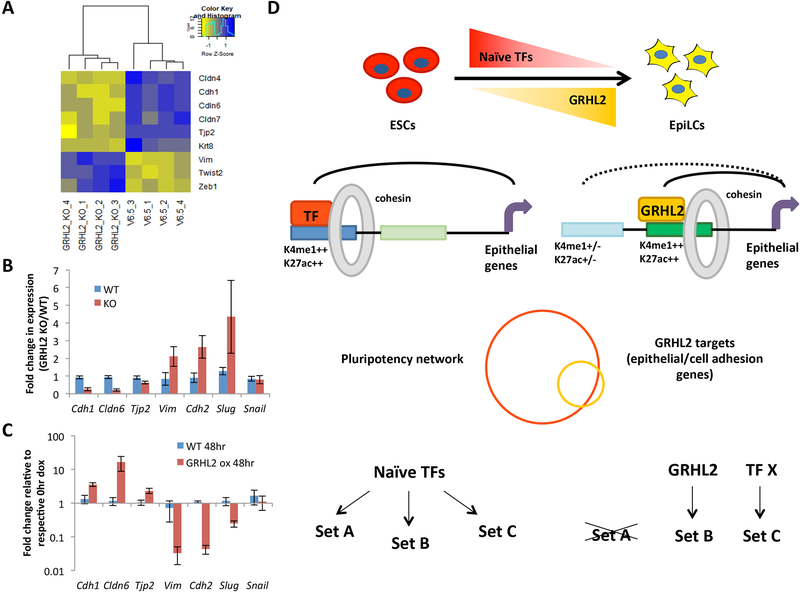
Figure 7. GRHL2 loss prevents maintenance of the epithelial expression program and results in EMT of EpiLCs.
A) Clustered heatmap of common epithelial and mesenchymal markers that are significantly changed (adjusted p<0.05) in 4 independent clones of GRHL2 KO EpiLCs vs WT (V6.5) EpiLCs. B) qPCR validation of expression changes for epithelial (Cdh1, Cldn6, Tjp2) and mesenchymal (Vim, Cdh2, Slug, Snail) markers in WT (V6.5) vs GRHL2 KO EpiLCs. Error bars show standard deviation for n=4 biological replicates. p<0.05 for all markers except Snail by t-test. C) Expression of epithelial and mesenchymal markers in WT and dox-inducible GRHL2 overexpressing ESCs treated with doxycycline for 0 or 48 hours as quantified by qPCR. Expression is normalized to respective 0 hr doxycycline samples for each cell line. Error bars indicate standard deviation for n=3 biological replicates. p<0.05 for all markers except Vim (p = 0.056) and Snail. D) A model where naïve-specific pluripotency factors including the KLF TFs regulate a broad repertoire of genes in ESCs, many that need to be maintained in the formative state. TFs up-regulated in the formative state activate new enhancers to maintain expression of these genes while the ESC-specific enhancers become inactivated. In the case of GRHL2, the target genes promote an epithelial state characteristic of both naïve and formative pluripotent cells. We speculate that other EpiLC-specific TFs regulate additional subsets of the larger naïve network.