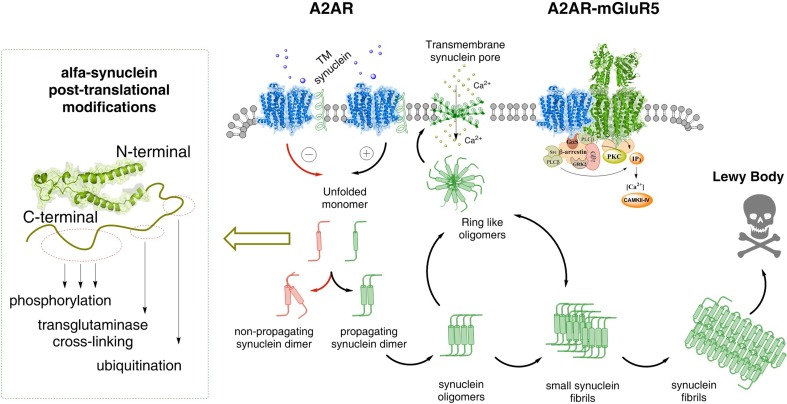
Fig. 3.
Possible molecular mechanism by which alpha-synuclein monomers/oligomers/synuclein fibrils can modulate the A2AR homo-heteroreceptor complexes and their balance in the plasma membrane. In the left part it is proposed that monomeric alpha-synuclein transmembrane (TM) peptides can become linked to the A2AR homoreceptor complex and modulate the A2AR function. Under the modulation of the monomeric alpha-synuclein peptides the A2AR antagonist may favor the formation of non-propagating alpha-synuclein dimers (pathway highlighted in red). Instead the A2A receptor agonist induced A2AR activation (pathway highlighted in green) may in the alpha-synuclein-A2AR complex produce signals that favor the propagation of alpha-synuclein dimers/oligomers into small and large synuclein aggregates that accumulate in Lewy bodies.In the far left several mechanisms are illustrated that can produce posttranslational modifications of alpha-synuclein. It involves changes in phosphorylation, transglutaminase cross-linking and ubiquitination and may play a role in the transformation of alpha-synuclein into propagating alpha-synuclein dimers. Ring-like synuclein oligomers can also be formed, which enter the plasma membrane and there produce beta sheet structures that associate and produce pores through which calcium ions may pass. In the A2AR–mGluR5 heteromer, shown as the coming together of two homodimers (A2AR homodimer in blue and mGluR5 homodimer in green), the signaling pathways are illustrated. Changes in the activity of protein kinases like PKA, PKC and calcium–calmodulin kinase II can have a role in the modulation of the synuclein aggregation process