Figure 1.
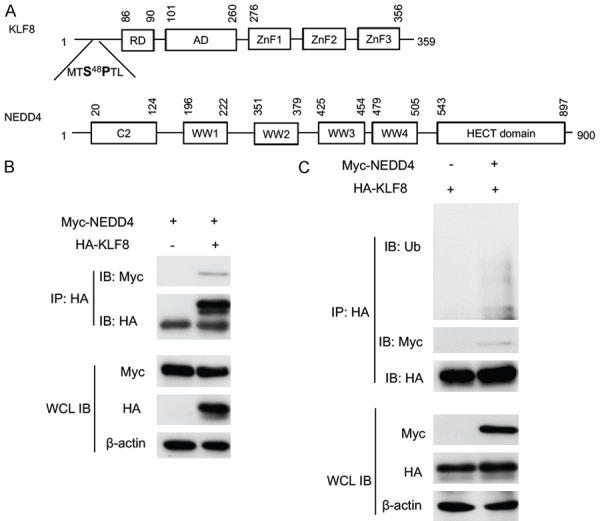
KLF8 interacts with and is ubiquitylated by NEDD4. A. The domain structure of KLF8 and NEDD4. Amino acid positions are numbered on the shoulders of the individual domains. Potential interacting S48P motif of KLF8 and WW domain of NEDD4 are highlighted. B. NEDD4 is co-IPed with KLF8. Myc-tagged NEDD4 was co-transfected with HA-tagged KLF8 in HEK293 cells. Whole cell lysates (WCL) were prepared 48 h later for IP with an anti-HA antibody for the KLF8 and co-IPed NEDD4 determined by subsequent immunoblotting (IB) with an anti-HA and anti-Myc antibody, respectively. C. KLF8 is ubiquitylated by NEDD4. NEDD4 was co-transfected with HA-KLF8 in HEK293 cells. KLF8 was IPed with an anti-HA antibody and ubiquitylated KLF8 was detected by immunoblotting the precipitates with an anti-ubiquitin antibody. Expression of the KLF8 and NEDD4 in the WCL was confirmed by IB with an anti-actin antibody serving as a loading control.
