Figure 2.
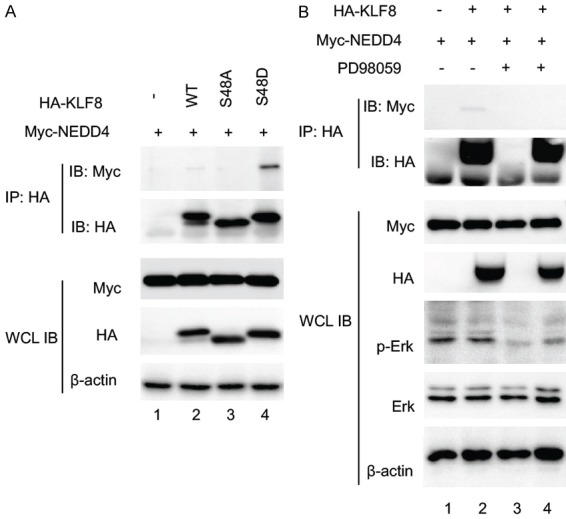
S48 phosphorylation by ERK is required for KLF8 to interact with NEDD4. A. The Serine 48 residue of KLF8 is responsible for the binding of NEDD4 and KLF8. HA-KLF8, wild-type or mutants indicated, was co-expressed with Myc-NEDD4 in HEK293 cells for 48 hours. Co-IP and IB were carried out similarly as described in Figure 1. B. ERK activity is required for the binding of KLF8 to NEDD4. Similarly treated HEK293 cells overexpressing Myc-NEDD4 and HA-KLF8 were treated with the MEK inhibitor PD98059 (50 mM) or DMSO for 30 min prior to cell lysate preparation for the similar co-IP assay. Effective inhibition of ERK activity was verified by IB, the WCL with an antibody against active ERK (p-Erk) and an antibody against total ERK (Erk). Expression of the KLF8 and NEDD4 was confirmed by immunoblotting the WCL with b-actin serving as a loading control.
