FIG 3.
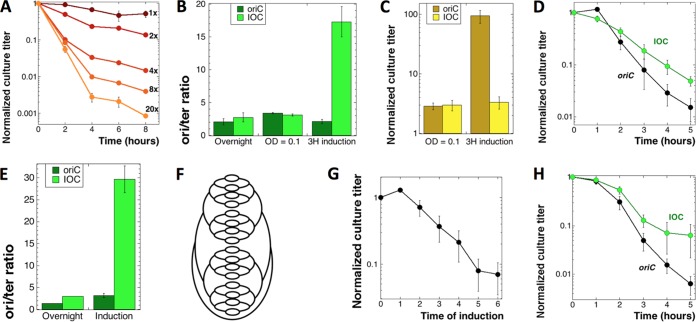
Driving CRC above the functional limit lessens TLD. Throughout this figure, “oriC” stands for our regular ΔthyA mutant (KKW58), while “IOC” stands for the ΔthyA mutant with the inducible origin construct (SRK290). (A) The extent of the dilution of stationary ΔthyA mutant cultures into fresh –dT medium determines the extent of subsequent TLD. 1× to 20×, fold dilutions into fresh medium. (B) ori/ter ratio of the oriC and IOC strains under induction condition 1. Cultures were grown overnight at 37°C in MOPS-CAA +dT medium, subcultured in the same medium, and grown to an OD600 of 0.1. At this time, IPTG was added, and induction was continued for 3 h before determination of the ori/ter ratio. (C) Culture titers upon induction under condition 1 (normalized to CFU at the time of subculture). (D) TLD kinetics of the IOC strain versus the thyA mutant under induction condition 1 (described for panel B). The cells were washed and T starved in MOPS-CAA medium without IPTG. Since during the induction the thyA culture became dense, it was diluted to an OD of 0.1 to 0.2 at the onset of starvation. (E) ori/ter ratios of the thyA and IOC strains grown under induction condition 2. Cultures of thyA and IOC strains were grown overnight at 37°C in MOPS-CAA +dT medium, subcultured in MOPS-CAA +dT medium with IPTG, and allowed to grow until the OD of IOC reached 0.1 to 0.3 and stopped increasing further. Incubation was continued for another 2 h, following which both cultures were harvested, and samples were collected to determine the ori/ter ratio. (F) The chromosomal scheme of IOC under induction condition 2 (CRC = 32). (G) Culture titers of the IOC strain upon induction under condition 2 (normalized to preinduction CFU). (H) TLD kinetics of the IOC mutant versus the thyA mutant under induction condition 2 (described for panel E). The cells were washed and starved in MOPS-CAA medium without IPTG.