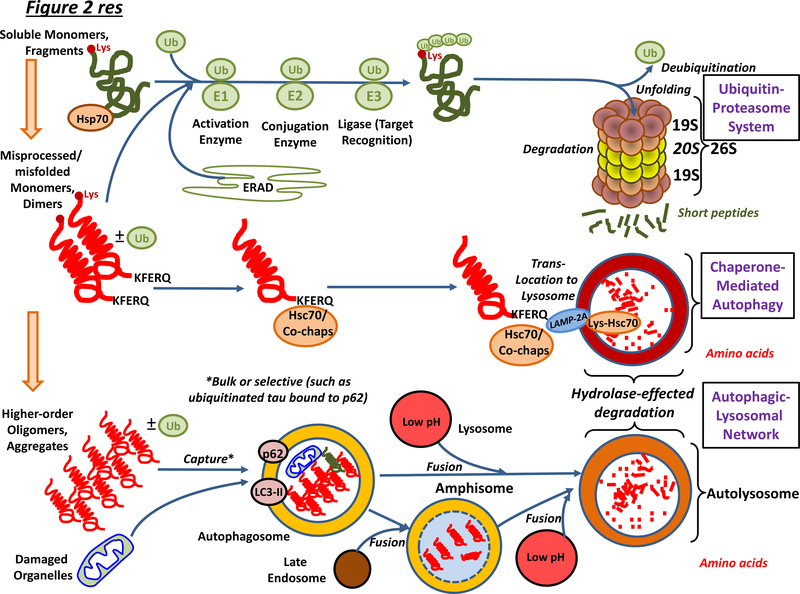
Figure 2 |. Overview of intracellular mechanisms for the elimination of neurotoxic proteins from neurons and other classes of cell in the brain.
Within neurons and other classes of cell, the UPS and CMA clear non-aggregated forms of neurotoxic protein, and the UPS also deals with substrates of endoplasmic reticulum-associated degradation (ERAD) of incorrectly-folded proteins. Proteins destined for the proteasome are poly-ubiquinated and guided to the proteasome by chaperones. They are deubiquinated by Rpn11 once committed to entering the proteosome pore: other deubiquitinases such as USP14 may rescue them before entry49. Unfolding is followed by degradation. The CMA operates on proteins bearing a KFERQ-like motif. This sequence is found in, for example, tau but not Aβ. Hsc70 recognises the KFERQ sequence and, together with co-chaperones, transports the protein to the LAMP2A receptor on lysosomes: LAMP2A then coordinates protein translocation into the lumen. The ALN is the major system for removing misfolded, higher-order, aggregated proteins as well as damaged organelles. Autophagosomes bearing cargo fuse with acidic lysosomes, leading to degradation of contents. In addition, some autophagosomes fuse with late endosomes. The resultant amphisomes then likewise fuse with lysosomes. See also Figure 3. Abbreviation not in main text: Co-chap, co-chaperone; Lys, lysine and Ub, ubiquitin,